...
Maleic anhydride.
| Names | |
|---|---|
| Related compounds | Maleic acid |
What is the color of maleic anhydride?
It is the acid anhydride of maleic acid and in its pure state is a colorless or white solid with an acrid odor. Maleic anhydride was traditionally manufactured by the oxidation of benzene or other aromatic compounds.
How do you make maleic anhydride?
Traditionally maleic anhydride was produced by the oxidation of benzene or other aromatic compounds. As of 2006, only a few smaller plants continue to use benzene. In both cases, benzene and butane are fed into a stream of hot air, and the mixture is passed through a catalyst bed at high temperature.
Is maleic anhydride a safe chemical?
Maleic anhydride is also a skin and respiratory sensitizer. Maleic anhydride is a low hazard profile chemical. Maleic anhydride rapidly hydrolyzes to form maleic acid in the presence of water and hence environmental exposures to maleic anhydride itself are unlikely.
What is the history of maleic anhydride?
Maleic anhydride as well as maleic and fumaric acids were first prepared in the 1830s. However, commercial manufacture did not begin until a century later. In 1933, the National Aniline and Chemical Co., Inc., used a process for producing maleic anhydride based on benzene oxidation using a vanadium oxide catalyst.

What functional groups are in maleic anhydride?
Using a chemical hydration reaction, maleic anhydride is derived from the compound maleic acid. The structure of maleic acid contains two carboxylic acid functional groups, which classifies maleic acid as a dicarboxylic acid functional group.
Is maleic anhydride aliphatic?
Maleic anhydride, 250 g | Aliphatic Building Blocks | Building Blocks for Synthesis | Organic & Bioorganic Chemicals | Chemicals | Carl Roth - International.
Is maleic anhydride cyclic?
It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor....ChEBI Namemaleic anhydrideChEBI IDCHEBI:474859DefinitionA cyclic dicarboxylic anhydride that is the cyclic anhydride of maleic acid.8 more rows•Mar 8, 2019
Is maleic anhydride an acid?
Maleic anhydride is a cyclic dicarboxylic anhydride that is the cyclic anhydride of maleic acid. It has a role as an allergen. It is a cyclic dicarboxylic anhydride and a member of furans.
Why is maleic anhydride a good dienophile?
Maleic anhydride is also a very good dienophile, because the electron-withdrawing effect of the carbonyl groups causes the two alkene carbons to be electron-poor, and thus a good target for attack by the pi electrons in the diene.
Why is maleic anhydride used?
Maleic anhydride is used in the production of unsaturated polyester resin as well as in the manufacture of coatings, pharmaceutics, agricultural products, surfactants, and as an additive of plastics.
Is maleic anhydride planar?
The maleic anhydride molecule is slightly non-planar, the oxygen atom within the five-membered ring lying 0-03 A from the plane of the other atoms.
What is the structure of maleic anhydride?
C4H2O3Maleic anhydride / Formula
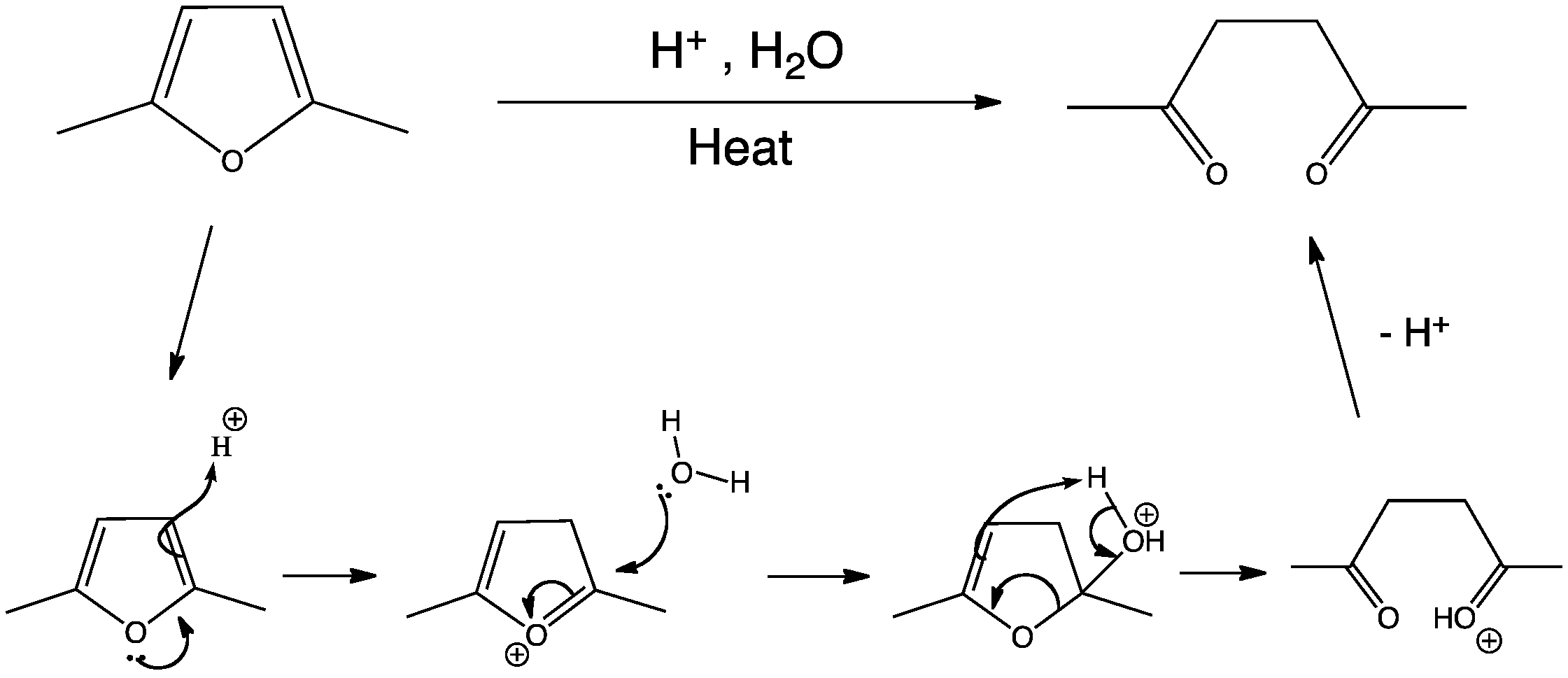
What happens when maleic anhydride reacts with water?
Reacts slowly with water to form maleic acid and heat. MALEIC ANHYDRIDE react vigorously on contact with oxidizing materials. Reacts exothermically with water or steam.
Is maleic anhydride toxic?
exposures may cause a build-up of fluid in the lungs (pulmonary edema), a medical emergency. ► Exposure to Maleic Anhydride can cause headache, dizziness, nausea and vomiting.
Does maleic anhydride dissolve in water?
A reactive, white solid compound that is used in the manufacture of polyester and alkyd resins. Maleic anhydride has needle-like crystals that dissolve readily in water to form Maleic acid.
How is maleic anhydride made?
Maleic anhydride is produced by oxidation of benzene or a C4 hydrocarbon such as butane in the presence of a vanadium oxide catalyst. Maleic anhydride can be converted to maleic acid by hydrolysis and to esters by alcoholysis.
What is the structure of maleic anhydride?
C4H2O3Maleic anhydride / Formula
Is maleic anhydride a liquid or solid?
Maleic anhydride (MA) is a clear liquid with a very unpleasant odor. The chemical intermediate product consists of the raw material benzene or n-butane. The downstream products are fumaric acid, malic acid and succinic acid, as well as butandiol. These are manufactured in an integrated production process.
Is maleic anhydride planar?
The maleic anhydride molecule is slightly non-planar, the oxygen atom within the five-membered ring lying 0-03 A from the plane of the other atoms.
What happens when maleic anhydride reacts with water?
Reacts slowly with water to form maleic acid and heat. MALEIC ANHYDRIDE react vigorously on contact with oxidizing materials. Reacts exothermically with water or steam.
What is maleic anhydride?
Maleic Anhydride. Maleic anhydride contains a double bond between the second and third carbons, which allow it to react with polyolefins in peroxide initiated processes (Sathe et al., 1994). From: Wood Composites, 2015.
Why is maleic anhydride used in polyalkene?
Maleic anhydride (MAH) is widely used for polyalkene functionalization due to its low tendency to homopolymerize and the formation of short grafts which allow it to keep most of the physical and mechanical properties of the support polymer. MAH is, however, bothered by too low a reactivity, as the anhydride group improverishes the double bond of its electrons. To compensate for this effect, higher amounts of peroxides are introduced for the melt grafting in extruders. According to this higher level of initiator, the grafting has to compete with nonegligible parasitic reactions like crosslinking (mainly with the PE) and chain degradation (PP). Many research groups are trying to overcome these problems by increasing the grafting efficiency, which allows a lower initiator concentration and reduces the risk of parasitic events.
Why is n-butane used in maleic anhydride?
n -Butane oxidation grew rapidly as the preferred process and is now dominant for maleic anhydride production, for three reasons: (1) benzene is a valued petrochemical feedstocks whereas the cost of n -butane is effectively that of a fuel; (2) the recognition of benzene as a carcinogen now requires the adoption of measures against its release in the workplace and in the environment; and (3) two of its carbon atoms are lost as carbon dioxide.
How does heating polyethylene in maleic anhydride solution generate polyethylene macroradicals?
Heating polyethylene in maleic anhydride solution generates polyethylene macroradicals by reaction of peroxide radicals with polyethylene and by reaction of polyethylene–maleic anhydride radicals with polyethylene chains (see Fig. 13.11 ). These macroradicals may set off the polymerization of maleic anhydride or join the developing chains of poly (maleic anhydride). The polypropylene-grafted maleic anhydride (PP-g-MAH) is formed by the same mechanism, only with the polymer chains being polypropylene instead. It acts as a bridge between the RS/RH and the polymer matrix where the polypropylene chains embed into the polymer matrix and maleic anhydride connects with hydroxyl groups in the fibers, as shown in Fig. 13.12.
How to dissolve maleic anhydride?
Pulverized maleic anhydride (6 g, 0.061 mole) is dissolved in 20 ml of ethyl acetate by gentle heating on a steam bath. Petroleum ether (20 ml) is added slowly to the solution, which is then cooled in an ice bath. To the cold solution is added 4.8 g (6 ml, 0.073 mole) of cyclopentadiene, and the resulting solution is swirled until the exothermic reaction subsides and the product separates. Recrystallization may be carried out in the reaction solvent by heating until dissolution occurs (steam bath) followed by slow cooling. The product has mp 164–165°, yield about 80%.
What is MAH associated with?
This orientation has been reinforced with the use of a ‘mixed monomer system’ CTC: MAH is associated with an electron donor comonomer which increases its reactivity and is involved in the grafting. A crowed of comonomers have been tested for this combined grafting reaction. Vinyl comonomers which copolymerize well with MAH and have reduced steric hindrance give better results, like alkenes, acrylates, styrene or α-methylstryrene. 107 The proposed classification is isobutylene > propylene > styrene = vinyl acetate, 108 but styrene 27,104,105,109,110 and, to a lesser extent, vinyl acetate and ethylene, are the most used.
When was maleic anhydride first made?
Maleic anhydride as well as maleic and fumaric acids were first prepared in the 1830s. However, commercial manufacture did not begin until a century later. In 1933, the National Aniline and Chemical Co., Inc., used a process for producing maleic anhydride based on benzene oxidation using a vanadium oxide catalyst.
What is maleic anhydride used for?
Maleic anhydride is used in the formulation of resins. Exposure to maleic anhydride may occur from accidental releases to the environment or in workplaces where it is produced or used.
What temperature does maleic anhydride melt?
Maleic anhydride appears as colorless crystalline needles, flakes, pellets, rods, briquettes, lumps or a fused mass. Melts at 113°F. Shipped both as a solid and in the molten state. Vapors, fumes and dusts strong irritate the eyes, skin and mucous membranes.
How long does it take for maleic anhydride to hydrolyze?
However, maleic anhydride hydrolyzes rapidly in water forming maleic acid with hydrolysis half-lives of 3.32 and 0.37 minutes at 0 and 25.1 °C respectively (3). Therefore, volatilization is not expected to be an important fate process in water or moist soils (SRC).
How long does maleic anhydride stay in the atmosphere?
Vapor-phase maleic anhydride will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals, ozone molecules and nitrate radicals with half-lives 11 days, 6.5 days and 8 hours, respectively. Atmospheric reaction with nitrate radicals is an important night time sink for maleic anhydride.
Is maleic anhydride an allergen?
Maleic anhydride is a cyclic dicarboxylic anhydride that is the cyclic anhydride of maleic acid. It has a role as an allergen. It is a cyclic dicarboxylic anhydride and a member of furans. Maleic anhydride is used in the formulation of resins. Exposure to maleic anhydride may occur from accidental releases to the environment or in workplaces ...
Does maleic anhydride affect fertility?
In two rat studies (90 days or 90 and 183 days), dietary administration of maleic anhydride resulted in nephritis. In a two-generation rat study with gavage administration, no effects on fertility were found, however the pregnancy rate of the control group was exceptionally low (50-70%).
What is maleic anhydride used for?
Maleic anhydride is used in the production of unsaturated polyester resin as well as in the manufacture of coatings, pharmaceutics, agricultural products, surfactants, and as an additive of plastics.
What is the formula for maleic anhydride?
TCC’s Maleic Anhydride is an organic compound with the formula C2H2 (CO)2O. It is the acid anhydride of maleic acid and in its pure state is a colorless or white solid with an acrid odor. Maleic anhydride was traditionally manufactured by the oxidation of benzene or other aromatic compounds.
Does maleic anhydride use n-butane?
As of 2006, only a few smaller plants continue to use benzene; due to rising benzene prices, most maleic anhydride plants now use n-butane as a feedstock. The chemistry of maleic anhydride is very rich, reflecting its ready availability and bifunctional reactivity. It hydrolyzes, producing maleic acid, cis-HOOC–CH=CH–COOH.
Is maleic anhydride a dienophile?
Maleic anhydride is a potent dienophile in Diels-Alder reactions. It is also a ligand for low-valent metal complexes, examples being Pt (PPh 3) 2 (MA) and Fe (CO) 4 (MA). Maleic anhydride dimerizes in a photochemical reaction to form cyclobutane tetracarboxylic dianhydride (CBTA).