
Is forming a chemical bond always an exothermic process?
Chemical bond formation is always an exothermic process and chemical bond breaking is an endothermic process. When bonds are formed the system loses energy and hence increases its stability (which is the ultimate motive). Since their is a decrease in energy, the energy lost is released as heat energy and thus it is an exothermic process.
Is freezing an endothermic or exothermic process?
Freezing, the transition from liquid to solid, is an exothermic process because energy is released in the form of heat. Since freezing/melting is a first-order phase transition, latent heat participates in this transition. The absorbed heat begins to destroy the intermolecular attraction of liquid freon, providing an endothermic evaporation process.
Is dissolving an endothermic or exothermic process?
The process of dissolving can be endothermic (temperature goes down) or exothermic (temperature goes up). When water dissolves a substance, the water molecules attract and “bond” to the particles (molecules or ions) of the substance causing the particles to separate from each other.
Is melting a metamorphic process?
When rock melts it is then considered igneous not metamorphic, but the rock next to the melted rock can be changed by the heat and become a metamorphic rock. The diagram above shows you where metamorphic rock (YELLOW ZONE) can be produced at a subduction zone.
Why is Respiration an Exothermic Reaction?
What is neutralization reaction?
What is an exothermic reaction?
What are some examples of combustion reactions?
What is the name of the exothermic reaction in which a fuel undergoes reduction when exposed to an?
What is the formula for glucose?
What happens when uranium is fissioned?
See 4 more
About this website
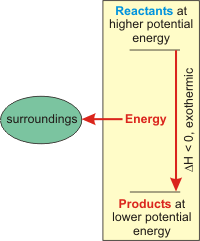
Is melting exothermic or endothermic?
endothermic processFinal Answer: Melting is an endothermic process because when heat absorbs then the physical state will change from solid to liquid.
Why is melting an endothermic reaction?
Explanation: Water is a higher energy state, as the liquid can rotate and vibrate while solid ice can only vibrate. This means for ice to turn into a higher energy state (water) it has to absorb energy, hence it is an endothermic process with respect to the system (surrounding temperature decreases).
Why is melting an exothermic process?
So, is melting exothermic or endothermic? Melting is an endothermic process as we need to apply external heat to a solid substance in order to make it melt. Heat is absorbed by the reactant species in the melting process and the change in enthalpy results out to be positive indicating the reaction to be endothermic.
Is melting always an endothermic reaction?
Melting is the phase change process in which heat is moved from the surroundings to the system to break the intermolecular force that holds the solid molecule together; thus, melting requires additional energy from the surrounding. Therefore, it is an exothermic process, not an endothermic reaction.
What type of reaction is melting?
The melting of ice absorbs heat, so it is an example of an Endothermic reaction.
When ice melts is it endothermic or exothermic?
endothermic reactionWhere an exothermic reaction releases heat, an endothermic reaction absorbs heat. One common endothermic reaction is ice melting.
What are the 3 exothermic processes?
Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes.
Is boiling exothermic or endothermic?
endothermicBecause we must add heat, boiling water is a process that chemists call endothermic.
What processes are exothermic?
Some examples of exothermic processes are: Combustion of fuels such as wood, coal and oil/petroleum. The thermite reaction. The reaction of alkali metals and other highly electropositive metals with water.
How can you tell if a reaction is endothermic or exothermic?
There are two methods for distinguishing between exothermic and endothermic reactions. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When energy is absorbed in an endothermic reaction, the temperature decreases.
Why is melting and evaporation endothermic?
This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. Thus, these phase changes are an example of an endothermic reaction.
Why is melting and evaporation endothermic?
This means that as you move from solid to liquid to gas, all accompanying phase changes require the input of heat. Thus, these phase changes are an example of an endothermic reaction.
Why are melting and boiling endothermic?
If heat is added to a substance, such as in melting, vaporization, and sublimation, the process is endothermic. In this instance, heat is increasing the speed of the molecules causing them move faster (examples: solid to liquid; liquid to gas; solid to gas).
What causes an endothermic reaction?
An endothermic reaction occurs when the temperature of an isolated system decreases while the surroundings of a non-isolated system gains heat. Endothermic reactions result in an overall positive heat of reaction (qrxn>0).
Why is melting and vaporization endothermic?
Solids, which are highly ordered, have the strongest intermolecular interactions, whereas gases, which are very disordered, have the weakest. Thus any transition from a more ordered to a less ordered state (solid to liquid, liquid to gas, or solid to gas) requires an input of energy; it is endothermic.
Define exothermic reaction.
An Exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light.
What is a Combustion example?
Combustion refers to the process where a substance burns in the presence of Oxygen, giving off heat and light in the process.
How do we use combustion?
Thermal energy derived from the combustion of either fossil fuels such as coal or oil or from renewable fuels such as firewood is extracted for a r...
What is the sign of enthalpy change for an exothermic reaction?
The change in enthalpy ( ΔH) for an exothermic reaction will be negative.
Give examples of exothermic reactions.
Combustion reaction, Neutralization reaction, and Respiration.
Examples of Endothermic and Exothermic Reactions
Endothermic reactions. Photosynthesis. Photosynthesis is the chemical reaction that takes place in green plants, which uses energy from the sun to change carbon dioxide and water into food that the plant needs to survive, and which other organisms (such as humans and other animals) can eat so that they too can survive. The equation for this reaction is:
11 Endothermic Reaction Example: Detailed Explanations
To know more please follow: Stereoselective vs Stereospecific: Detailed Insights and Facts Melting of Ice to form water. Melting of ice to form water is an example of phase change reaction and it proceeds by endothermic pathway. Ice melts at 273 K or above 273K temperature. For melting of ice at 273K temperature, heat absorbed by system is equal to the latent heat (80cal/g) and for melting ice ...
Why is Respiration an Exothermic Reaction?
Respiration is the process by which humans take in oxygen and give out carbon dioxide. The chemical equation of this process can be represented as follows:
What is neutralization reaction?
Neutralization reactions are chemical reactions wherein the reactants include an acid and a base, which go on to combine to yield a salt and water. These reactions neutralize the pH of the reacting species and hence are named after this neutralizing feature. The standard enthalpy change (ΔH) for ...
What is an exothermic reaction?
An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative.
What are some examples of combustion reactions?
Another example of a combustion reaction is the combustion of glucose, given by the following reaction: C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
What is the name of the exothermic reaction in which a fuel undergoes reduction when exposed to an?
Combustion. Commonly referred to as burning, combustion is an exothermic reaction in which a fuel undergoes reduction when exposed to an oxidizing agent (which is usually the oxygen present in the atmosphere) and forms an oxidized product. These reactions give out good amounts of energy in the form of heat, but also form some byproducts such as ...
What is the formula for glucose?
In the reaction given above, it can be noted that C6H12O6 is the formula for glucose, which combines with the oxygen inhaled by humans to form carbon dioxide, water, and energy.
What happens when uranium is fissioned?
Nuclear Fission of Uranium-235 1 The nuclear fission of one atom of uranium-235 releases more than 2.5 million times the energy that is produced from the combustion of coal. 2 The fission of one atom can cause a chain reaction in which the neutron from the fission of one uranium-235 atom can strike another atom, leading to its nuclear fission. 3 This isotope of uranium is used in nuclear power plants, in which the fission reaction is controlled with the help of control rods that are capable of absorbing neutrons.
What is Riti Gupta's degree?
Riti Gupta holds a Honors Bachelors degree in Biochemistry from the University of Oregon and a PhD in biology from Johns Hopkins University. She has an interest in astrobiology and manned spaceflight. She has over 10 years of biology research experience in academia. She currently teaches classes in biochemistry, biology, biophysics, astrobiology, as well as high school AP Biology and Chemistry test prep.
Why do you need to put water over a flame?
You need to put the water over a flame in order to add heat to the system and have the water boil in order to make water vapor. This input of energy is also enough to overcome the attractive forces that hold the particles together.
How to make liquid water into ice?
In order to make liquid water into ice you must put the water into a cold environment so that heat leaves the water. Only then will the water freeze.
What is the name of the phase change?
Phase Change Name: Deposition. Phase: Gas to solid. Energy Change: Exothermic. Example: Formation of frost. A good way to remember all of these is that opposite phase changes have opposite energy needs. If you know that from solid to liquid to gas requires the addition of heat (endothermic), that means you know that going from gas to liquid ...
What allows water to melt?
The molecules of water in the piece of ice are not moving much until the water starts to melt. What allows the water to melt? Well, it's an addition of heat.
Is frost an endothermic or exothermic reaction?
All of these changes in the phase of water are accompanied by either an input or output of heat, so they are either an endothermic reaction or an exothermic reaction.
Is a phase change endothermic or exothermic?
Here is how to classify the phase changes as endothermic or exothermic: A good way to remember all of these is that opposite phase changes have opposite energy needs. If you know that from solid to liquid to gas requires the addition of heat (endothermic), that means you know that going from gas to liquid to solid requires the removal of heat ...
What is exothermic reaction?
Updated January 14, 2019. An exothermic reaction is a chemical reaction that releases heat and has a negative enthalpy (-ΔH) and positive entropy (+ΔS).. These reactions are energetically favorable and often occur spontaneously, but sometimes you need a little extra energy to get them started.
Why are exothermic reactions so interesting?
Exothermic reactions make interesting and exciting chemistry demonstrations because the release of energy often includes sparks, flame, smoke, or sounds, in addition to heat. The reactions range from safe and gentle to dramatic and explosive.
What is hot ice?
Hot ice is what you get when you solidify sodium acetate from a supercooled solution. The resulting crystals resemble water ice, except they are hot instead of cold. It's a fun example of an exothermic reaction. It's also one of the common reactions used to make chemical hand warmers .
Why is it called the barking dog?
It's called the Barking Dog because that is what the chemical reaction sounds like. The "barking dog" reaction is a favorite exothermic chemistry demonstration because it emits a loud 'woof' or 'bark', similar to that of a dog.
What happens when you react sulfuric acid with sugar?
Dehydrating the sugar pushes out a steaming column of carbon black, plus it makes the entire room smell like burnt marshmallows.
What happens if you believe in "go big or go home"?
If you believe "go big or go home," then try performing the thermite reaction inside a block of dry ice. This amplifies the process and may even produce an explosion.
Can exothermic reactions burst into flame?
Exothermic reactions often burst into flame without the need for a match or other ignition source. Some exothermic chemical reactions spontaneously burst into flame without needing the help of a lit match. There are several ways to make a chemical fire -- all terrific demonstrations of exothermic processes.
Why is Respiration an Exothermic Reaction?
Respiration is the process by which humans take in oxygen and give out carbon dioxide. The chemical equation of this process can be represented as follows:
What is neutralization reaction?
Neutralization reactions are chemical reactions wherein the reactants include an acid and a base, which go on to combine to yield a salt and water. These reactions neutralize the pH of the reacting species and hence are named after this neutralizing feature. The standard enthalpy change (ΔH) for ...
What is an exothermic reaction?
An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative.
What are some examples of combustion reactions?
Another example of a combustion reaction is the combustion of glucose, given by the following reaction: C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
What is the name of the exothermic reaction in which a fuel undergoes reduction when exposed to an?
Combustion. Commonly referred to as burning, combustion is an exothermic reaction in which a fuel undergoes reduction when exposed to an oxidizing agent (which is usually the oxygen present in the atmosphere) and forms an oxidized product. These reactions give out good amounts of energy in the form of heat, but also form some byproducts such as ...
What is the formula for glucose?
In the reaction given above, it can be noted that C6H12O6 is the formula for glucose, which combines with the oxygen inhaled by humans to form carbon dioxide, water, and energy.
What happens when uranium is fissioned?
Nuclear Fission of Uranium-235 1 The nuclear fission of one atom of uranium-235 releases more than 2.5 million times the energy that is produced from the combustion of coal. 2 The fission of one atom can cause a chain reaction in which the neutron from the fission of one uranium-235 atom can strike another atom, leading to its nuclear fission. 3 This isotope of uranium is used in nuclear power plants, in which the fission reaction is controlled with the help of control rods that are capable of absorbing neutrons.
