How to write the name for N2O?
Nitrous oxide, commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula N 2 O.At room temperature, it is a colourless non-flammable gas, with a slight metallic scent and taste.
What are the rules for naming compounds?
- Alkanes - saturated hydrocarbons The names of the straight chain saturated hydrocarbons for up to a 12 carbon chain are shown below. ...
- Alkyl halides The halogen is treated as a substituent on an alkane chain. ...
- Alkenes and Alkynes - unsaturated hydrocarbons Double bonds in hydrocarbons are indicated by replacing the suffix -ane with -ene . ...
Is 2O2 a compound or an element?
Oxygen is an element. A compound is two or more elements chemically combined. A mixture is two or more elements physically combined (ie. just mixed together). Oxygen gas is made of molecules containing only oxygen. Normal atmospheric oxygen is O 2 and ozone is O 3. So, it certainly isn't a compound.
What is the chemical name for N2O?
Uses of Nitrous Oxide (N2O)
- It is used rocket motor as an oxidizer
- It is used as a food additive as an aerosol spray propellant
- It is used in the manufacturing of semiconductors
- It is used in the medical field as an analgesic and anaesthetic
- It is used as a flavouring ingredient
- It is used in car racing as a fuel additive
- It is used in dentistry
- It is used to manufacture chemicals

What type of compound is N2O2?
nitrogen oxideDioxohydrazine is a nitrogen oxide.
What is the proper name for N2O2?
Nitrous oxideNamesChemical formulaN 2OMolar mass44.013 g/molAppearancecolourless gasDensity1.977 g/L (gas)57 more rows
How N2O2 is formed?
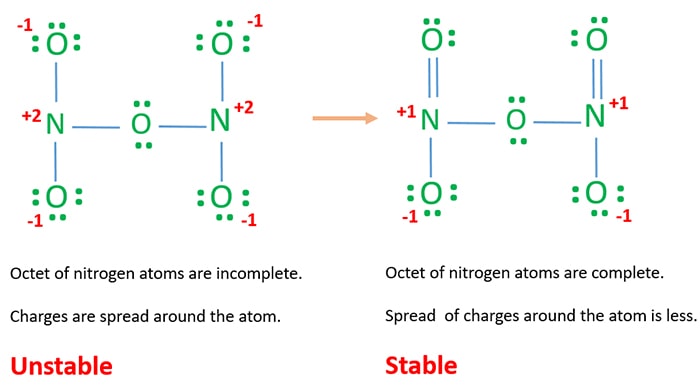
It is formed by adding pounded carbonate of ammonia to pure nitric acid, which is concentrated may be previously diluted with half its bulk of water so long as there is effervescence and a small excess of the carbonate may be left at the end in the liquor.
Is dinitrogen dioxide a covalent compound?
The covalent bonding pattern O=N–N=O. (a non-cyclic dimer of nitric oxide (NO)) is predicted to be the most stable isomer based on ab initio calculations and is the only one that has been experimentally produced....Dinitrogen dioxide.IdentifiersMolar mass60.012 g·mol−112 more rows
Is N2O2 empirical or molecular?
Molecular nitrogen;molecular oxygen | N2O2 - PubChem.
What is the molecular geometry of N2O2?
Nitrous oxide is linear.
Is nitrous oxide a molecule?
Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (•N=O. or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.
Is nitrous oxide an ionic compound?
Nitrous oxideNitrous oxide / IUPAC ID
Is n2o2 linear?
Shape of N2O molecule around center atom Number of lone pairs and number of σ bonds around the center atom are important for finding the shape of a molecule. Due to two sigma bonds and zero lone pairs around center atom (nitrogen), shape of N2O molecule is linear.
How do you name a molecular compound?
SummaryA molecular compound is usually composed of two or more nonmetal elements.Molecular compounds are named with the first element first and then the second element by using the stem of the element name plus the suffix -ide. Numerical prefixes are used to specify the number of atoms in a molecule.
Is CO2 covalent or molecular?
Carbon and oxygen are non-metals, thus we know carbon dioxide is a covalent compound.
Is CO2 a covalent molecular compound?
As per the definition, an ionic compound is a compound that is mostly formed between a metal atom and a non-metal atom. Meanwhile, CO2 is a compound that is formed between two non-metal atoms (carbon and oxygen) thus giving it a covalent nature.