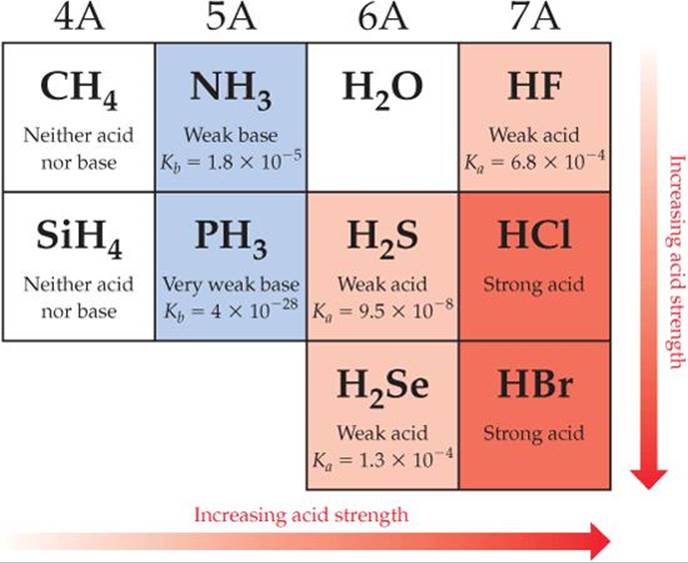
Is NH3 an acid or base according to Lewis?
However, according to Lewis’s theory of acids and bases, NH3 due to the presence of a lone pair of electrons is considered as a Lewis base. What is NH3 (Ammonia)?
Is NH4+ (ammonium ion) an acid or base?
Therefore, we can say NH 4+ is a Bronsted-Lowry acid. As per the above reaction, NH 4+ act as Bronsted-Lowry acid and release a proton to become NH 3, and NH 3 acts as Bronsted-Lowry base, as it is capable of accepting the proton from water to form ammonium ion again. Is NH4+ (Ammonium ion) is strong or weak acid?
Is NaOH an acid or a base?
Some compounds, like HCl or NaOH behave predictably as acids or bases, while others, like acetic acid, behaves like an acid to weak acids/weak bases, but as a very weak base toward sulfuric acid. , Anything about chemistry.
What happens when NH3 acts as a base?
What is NH3 gas?
What is the chemical formula for ammonia?
What is the reaction of NH3 and Lithium?
What is the reaction of NH3?
How many electrons does nitrogen have?
Why does NH3 mix with water?
See more
Is NH a strong or weak base?
weak baseNH4Cl is an example of an acid salt. The molecule NH3 is a weak base, and it will form when it can, just like a weak acid will form when it can. So there are two general rules: If an ion derives from a strong acid or base, it will not affect the acidity of the solution.
Is NH4+ an acid-base or both?
Yes, NH4+ is an acid. It has a pH of around 5.5 and is also capable of donating a proton in an aqueous solution. NH4+ ions do not dissociate completely in an aqueous solution and therefore NH4+ is considered as a weak acid. NH4+ is the conjugate acid of base Ammonia (NH3).
Why NH4 is a base?
It's a cation,not acid and base. NH4 molecule doesn't exist while NH4+ ion does exist and Ammonium ion NH4+ reacts with hydroxide ion OH- to form ammonium hydroxide which is base.
Why is NH4 an acid and NH3 a base?
NH4+ has a proton to donate. NH4+ H+ + NH3. H2SO3 also has protons it is able to donate, thus making it an acid. NH3 is a base because this nitrogen has a lone pair that is capable of accepting a proton.
Why is NH3 is a base?
In ammonia, the nitrogen atom has a lone pair of electrons that can be quickly donated to the necessary Lewis acid. Ammonia will thus serve as a Lewis base. Ammonia, NH3, is a Lewis base and has a lone pair. It will donate electrons to compounds that will accept them.
Is NH4+ hard acid?
Notice that hard acids are usually cations of electropositive metals; consequently, they are relatively nonpolarizable and have higher charge-to-radius ratios....Hard and Soft Acids and Bases.AcidsBaseshardH+NH3, RNH2, N2H4Li+, Na+, K+H2O, ROH, R2OBe2+, Mg2+, Ca2+, VO2+OH−, F−, Cl−, CH3CO2−Al3+, Sc3+, Cr3+CO32−5 more rows•Sep 16, 2020
Why is NH4+ weak acid?
0:102:43Is NH4+ a strong or weak acid (ANS: definitely weak!) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSome strong acids HCl HBR H I H no. 3 h2so4 and maybe even hclo4. Anything that's not on this listMoreSome strong acids HCl HBR H I H no. 3 h2so4 and maybe even hclo4. Anything that's not on this list is generally going to be a weak acid. But I'll also prove it to you NH. 4 plus when it reacts with
Is NH4+ A conjugate acid or base?
NH4+ is the conjugate acid of the base NH3.
Is NH4 a strong base?
NH3 and NH4+ are weak bases and acids that may be relatively "near the middle" on a scale showing the relative strengths of acids and bases.
Is ammonium ion a base?
Both ammonia is a weak base and ammonium ion is a weak acid.
Why is NH4+ more acidic than NH3?
NH4+ acts as a bronsted Lowry acid and donates an H to become NH3, and NH3 acts as a bronsted lowry base and accepts an H. In this pair, NH4+ is the acid and NH3 is the base, so NH4+ is the stronger acid. Second, more positively charged molecules/atoms are more acidic.
Which is more stable NH4 or NH3?
The basic rule to determine the stability of a compound is that neutral molecules will always be more stable than any of their ions.
Is NH4 a Buffer?
In chemistry, a solution containing an almost equal concentration of acid as well as a base is referred to as a buffer.
Why (ammonia) NH3 is a base?
A base is defined as a proton acceptor or lone pair donor. When NH3 dissolves in water, it will accept the H+ ion from the water and gets converted into conjugate acid (NH4+) and produces hydroxide ions (OH–).
Is Ammonia (NH3) a strong or weak base?
To know whether Ammonia (NH3) is a strong base or weak, you must know the basic difference between a strong base or a weak base.
Is Ammonia (NH3) also act as acid?
So, Is NH3 also act as an acid? Ammonia (NH 3) can act acid only in one condition when the reacting compound is more basic than it e.g. OH –. This is because a stronger base more dissociates in an aqueous solution and strongly accepts the proton.
Why NH3 act as a Lewis base?
Lewis’s theory is a very important acid-base theory to check whether a compound (NH3) is acid or base?
What is the conjugate acid of NH3?
In technical terms, Compounds differentiated from each other by a single proton (H+) are said to be Conjugate acid-base pairs.
How to know if NH3 is acid or base practically?
Till now we learn how to know if compound acid or not theoretically. To know practically, one of the easiest ways to use litmus paper.
Uses of Ammonia
Ammonia is considered a building block for the synthesis of many pharmaceutical products.
What is the difference between Lewis acid and Lewis base?
Lewis acid is simply acids that have the tendency of “accepting the lone pair of electrons” whereas Lewis base has a tendency of “donating the lone pair of electrons”. ⇒ Lewis acid → lone pair acceptor. ⇒ Lewis base → lone pair donator.
How is ammonium formed?
Ammonium is formed when NH 3 accepts the proton and forms a dative covalent bond with hydrogen. It is a polyatomic ion with a positive charge and contains no lone pair of electrons. In this article, we discussed the acid-base nature of NH 4+ .
Which molecule has a lone pair on the central atom that is capable of accepting the proton?
Whereas NH3 molecule has lone pair on the central atom that capable of accepting the proton and as per Bronsted-Lowry base “a base is capable of accepting the protons”. Image source: An acid is something that capable of donating the proton when dissolved in an aqueous solution.
Is ammonium ion an acid or base?
This theory tells if any compound can donate or release the proton when dissolved in an aqueous solution, qualify as acid and if any compound can accept the proton when dissolved in an aqueous solution, qualify as the base. So, in the case of ammonium ion, when dissolved in water, it ready to “give off” the proton.
Is ammonium an acid?
The ammonium ion is an acid in nature as it donates the proton when reacts with strong bases. However, it is a very weak acid having a pH value of 5.5 i.e. near to neutral in the acid-base scale. The proton donating ability of NH 4+ makes it mildly acidic. Name of Molecule.
Is ammonium cation soluble in water?
It is toxic to most of the crop species but it is an important source of nitrogen for those species which growing on hypoxic soils. Simple salts of ammonium ions are soluble in water. ...
Is ammonium ion strong or weak?
This part is really confusing to figure out what type of nature an ammonium ion can adopt “strong or weak”. To understand this we should dive into the concept of strong or weak acids. A strong acid is an acid that tends to dissociate fully or 100% ionized in an aqueous solution leaving no parts of it in solution.
What does acid plus base mean?
Traditionally an acid plus a base produces a salt plus water. Now if you are talking about unknown substances then it’s more difficult. Simple pH paper can tell you if the starting products are acids or bases in solution as well as indicate whether the end products have changed in pH.
What is Lewis acid?
Lewis Acid: Lewis acid is a compound or an ion which receives a pair of electron. Lewis Base: Lewis base is a compound or an ion which gives a pair of electron. Generally the positive ions generally known as the cations and compounds which have incomplete d orbital(non metalic oxide) are lewis acid.
Is NH2 cationic or acidic?
MahaDev. , Anything about chemistry. Answered 1 year ago. NH2 is acidic in nature as NH2 is a group is it is a functional group which is generally present in the form of NH2 plus that is cationic form NH2 Plus shows the more tendency to accept electrons which makes it acidic in nature as the acid the tendency to accept electron.
Is NaOH a weak base?
Some compounds, like HCl or NaOH behave predictably as acids or bases, while others, like acetic acid, behaves like an acid to weak acids/weak bases, but as a very weak base toward sulfuric acid. Find, evaluate and source engineering materials online.
Which ions have an incomplete d orbital?
Generally the positive ions generally known as the cations and compounds which have incomplete d orbital (non metalic oxide) are lewis acid. On the other hand compounds having one or more than one lone pair electon is capable of donating a pair of electrons and is called lewis base. For example:
Is NH2 an acid or base?
NH2 is neither an acid nor a base: it is an unstable radical. However, the NH2 radical, like in the compounds H-NH2 (more commonly writtten as NH3, ammonia) or methylamine, CH3NH2 behaves as a base toward acidic compounds.
Which is more basic NH or NH2?
A simpler way to put it: the conjugate base of an amine will always be a stronger base than the amine itself. Compare ammonia, (NH 3 ) with its conjugate base, the amide anion NH 2 (-). The amide anion is stronger base by far (pK a H of 38, versus pK a H of 9).
Is NH2 or NH more acidic?
Water is neutral whereas ammonia is a weak base (even less acidic). Stronger conjugate acids have weaker conjugate base and vice versa . Therefore, NH2- is a stronger base .
Which of the two is more basic and why nh2ch3 or NH2?
NH2 bcz..it has lone pair and can easily donate them and act as a Lewis Base. . Nitrogen has more basicity than carbon . in case of , Basicity increase left to right and top to bottom in periodic table . This discussion on Which is more basicity cH3 or NH2.
Which is a stronger base NH3 or NH2?
base of NH3 acting as an acid is NH2 ^-. This makes NH2 ^- a strong base . ... When you think about the relative strengths of acids and bases and acid- base reactions you should think of them as a competition for the H+ ion. A substance that readily donates a proton to H2O is considered and acid in an aqueous solution.
Which is more basic NH or NH2?
A simpler way to put it: the conjugate base of an amine will always be a stronger base than the amine itself. Compare ammonia, (NH 3 ) with its conjugate base, the amide anion NH 2 (-). The amide anion is stronger base by far (pK a H of 38, versus pK a H of 9).
Are NH bonds acidic?
Generally, O-H hydrogens are more acidic than N-H , which in turn are greater than C-H bonds. However...
Is NH basic or acidic?
Ammonium is the acid , donating the proton to water (Bronsted-Lowry definition) by accepting two electrons (Lewis definition) from water's oxygen.
What happens when NH3 acts as a base?
When NH3 acts as a base, it will donate its lone pair to a proton H+ and form its conjugate acid NH 4+ whereas when NH3 acts as an acid, it can give out H+ ion and forms a conjugate base as NH2-. Reactions are given below: (Acting as a Lewis Base) NH3 + H+ ——-> NH4+. (Acting as a Lewis Acid) NH3 ——–> NH2- + H+.
What is NH3 gas?
What is NH3 (Ammonia)? NH3 (Ammonia) is a non-flammable colorless gas that is lighter than the air. It has a very strong bad odor and considered a pungent-smelling gas due to its production by bacterial decomposition of urea.
What is the chemical formula for ammonia?
Ammonia’s chemical formula is NH3 and has a trigonal pyramidal shape with a Nitrogen atom on the pyramid top and 3 hydrogen atoms at the 3 base corners. The atomic number of Nitrogen is 7 and 5 electrons in its valence shell. This means that after the formation of 3 bonds with Hydrogen, Nitrogen carries a lone pair of electron.
What is the reaction of NH3 and Lithium?
It can lose H+ ion and form Amides (NH2-). One of the examples of such reaction is when Lithium reacts with NH3 to form Lithium Amide. (NH3 acting as a weak acid) Li + NH3 ——-> LiNH2 + H2.
What is the reaction of NH3?
NH3 undergoes exothermic combustion to produce Nitrogen gas and water vapor. Following is the combustion reaction of NH3. NH3 + O2 ——-> N2 + H2O ( enthalpy change of this reaction is −1267.20 kJ/mol) Although Nitrogen oxides are unstable with respect to N2 still we can form Nitrogen oxides with the help of some catalysts.
How many electrons does nitrogen have?
Nitrogen has one lone pair of electrons left after sharing 3 electrons to form bonds with 3 hydrogen atoms. This trigonal pyramidal shape provides the molecule with a dipole moment and makes it a polar molecule. Due to the presence of a lone pair, it has the ability to form hydrogen bonds in water.
Why does NH3 mix with water?
When put in water, NH3 readily mixes with water due to its polar nature and ability to form hydrogen bonds in water. It helps in the dissociation of H2O molecules in (Hydrogen ions) H+ and (Hydroxyl ions) OH- ions and forms bonding with H+ ions.