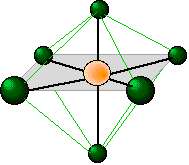
Is an octahedral molecule non-polar?
The molecule is non-polar since it is a symmetrical shape. Here are some examples of Octahedral-shaped molecules: What contributes to this shape?
What is an octahedral shape?
The Octahedral shape is a type of shape which a molecule takes form of when there are 6 bonds attached to a central atom with 4 on the same plane. There are no lone pairs attached to it.
Is a tetrahedral bond polar or nonpolar?
Tetrahedral is polar depending on geometry and can also be nonpolar. The three factors governing polarity are shape, electronegativity and dipole moment . All this concepts have been explained in detail in the sections followed . What type of bond is a tetrahedral?
What is the bond angle between the bonds in an octahedral molecule?
The bond angle between the bonds is exactly 90 degrees. The molecule is non-polar since it is a symmetrical shape. Here are some examples of Octahedral-shaped molecules:
Why do polar molecules have polar bonds?
What is the net dipole of a polar molecule?
Are all the octahedral are NonPolar?
Since there is an atom at the end of each orbital, the shape of the molecule is also octahedral....Octahedral.Shape:octahedralPolar/NonPolar:NonPolarHybridization:sp3d2Example:SF62 more rows•Aug 21, 2020
Is octahedral square pyramidal polar?
Hence the shape of the molecule is square pyramidal and the geometry is octahedral. This molecule is polar because the dipole moment due to the lone pair and the bond pairs does not cancel each other.
Is octahedral planar?
Is octahedral molecule planar? No, an octahedral molecule has two planes: an equatorial plane and an axial plane. The central atom and four of the terminal atoms lie in the equatorial plane. The central atom and the other two-terminal atoms lie in the axial plane.
Is tetrahedral polar or NonPolar?
Notice that a tetrahedral molecule such as CCl4 is nonpolar. However, if the peripheral atoms are not of the same electronegativity, the bond polarities don't cancel and the molecule becomes polar, as in CH3Cl. Figure 4.5.
Is octahedral linear polar or nonpolar?
POLARITY: POLAR - The lone pair electrons throw off the perfectly cancelling symmetry of the six octahedral regions thus making the overall molecule polar. The two lone pairs on this electronic geometry have to be 180° apart which forces the molecular geometry to be square planar.
Is octahedral square planar polar or nonpolar?
NonPolarThe shape of the orbitals is octahedral. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. The remaining four atoms connected to the central atom gives the molecule a square planar shape....Square Planar.Shape:square planarPolar/NonPolar:NonPolarHybridization:sp3d2Example:XeF42 more rows•Aug 21, 2020
Is octahedral planar or nonplanar?
Because of the high symmetry of the octahedral arrangement, all six positions are equivalent, so it does not matter in which position in the drawing we put the lone pair. The remaining four atoms connected to the central atom give the molecule a square planar shape.
What is the shape of octahedral?
The Octahedral Shape of Molecules contains eight faces. It has two square pyramids back to back, each square pyramid with four faces. That's why this is known as octahedral. It has the prefix octa which means eight.
Is tetrahedral and octahedral same?
Let us see some differences between tetrahedral and octahedral voids. Tetrahedral voids are unoccupied empty spaces present in substances having a tetrahedral crystal system. Octahedral voids are unoccupied empty spaces present in substances having an octahedral crystal system.
Is tetrahedral always non-polar?
A tetrahedral molecule can be polar or nonpolar. It all depends on symmetry and electronegativity concept. A molecule that is symmetrical and the atoms attached to the central atom have almost same electronegativity, then the molecule will be nonpolar.
Why tetrahedral is non-polar?
If there are no lone pairs then the molecular geometry matches the electronic and is tetrahedral. The base bond angle is 109.5° and there is no reason to tweak the bond to another value. POLARITY: NON-POLAR - As long as all four positions are the same, then the molecule cannot be polar due to perfect symmetry.
Why tetrahedral structures are non-polar?
Any 100% symmetrical tetrahedral molecule will be nonpolar. Tetrahedral molecules have no nonbonding electron pairs and all identical bond angles. Therefore, the only way they can be asymmetric is if one atom is different from the rest.
Is square pyramidal molecular geometry polar?
0:012:01Square Pyramidal Molecular Geometry/Shape and Bond AnglesYouTubeStart of suggested clipEnd of suggested clipAnd then we have the one lone pair so six total things and one of them is a lone pair that gives usMoreAnd then we have the one lone pair so six total things and one of them is a lone pair that gives us a square pyramidal molecular geometry a good example of this is clf5.
Are pyramidal polar or nonpolar?
Lewis Structures and the Shapes of MoleculesFormula3D Structure Shape Polarity1.CH4tetrahedral nonpolar2.NH3trigonal pyramidal polar3.H2Obent polar4.H3O+trigonal pyramidal charged2 more rows
Is sf6 a polar molecule?
According to VSEPR theory, a central atom with 6 covalently-linked peripheral atoms and zero non-bonding pairs, results in an octahedral molecular geometry. Each S-F bond is polar because fluorine is more electronegative. However, the molecule itself is non-polar, due to this geometry.
Are all pyramidal molecules polar?
Answer and Explanation: E.g., Ammonia is a trigonal pyramidal molecule with three hydrogens, one lone pair surrounding the central nitrogen atom, and it is a polar molecule. Thus, all trigonal pyramidal molecules are polar.
Why is the octahedral shape nonpolar?
The octahedral shape is almost always non polar because it is symmetric, causing the dipole moments to cancel one another. Hope this helps!
Is an octahedral a nonpolar molecule?
No. The octahedral shape is always nonpolar, because the shape is symmetrical. When a molecule has a symmetrical shape, the dipoles cancel out and the molecule in nonpolar.
How many edges does an octahedron have?
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces, twelve edges, and six vertices. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex .
What is an octahedron called?
Using the standard nomenclature for Johnson solids, an octahedron would be called a square bipyramid. Truncation of two opposite vertices results in a square bifrustum . The octahedron is 4-connected, meaning that it takes the removal of four vertices to disconnect the remaining vertices.
How many orthogonal projections does an octahedron have?
The octahedron has four special orthogonal projections, centered, on an edge, vertex, face, and normal to a face. The second and third correspond to the B 2 and A 2 Coxeter planes .
What is the octahedron with center coordinates?
In an x – y – z Cartesian coordinate system, the octahedron with center coordinates ( a, b, c) and radius r is the set of all points ( x, y, z) such that
How many vertices does a polyhedron have?
The following polyhedra are combinatorially equivalent to the regular polyhedron. They all have six vertices, eight triangular faces, and twelve edges that correspond one-for-one with the features of a regular octahedron.
What is the octahedron?
Geometric relations. The octahedron represents the central intersection of two tetrahedra. The interior of the compound of two dual tetrahedra is an octahedron, and this compound, called the stella octangula, is its first and only stellation.
Which Platonic solid has mirror planes that do not pass through any of the faces?
The octahedron is unique among the Platonic solids in having an even number of faces meeting at each vertex. Consequently, it is the only member of that group to possess mirror planes that do not pass through any of the faces.
What contributes to this shape?
The only thing that contributes to this shape are the six bonds which the central atom is attached to. There are no lone pairs of electrons which would repel with the surrounding atoms and provide a different bond angle and shape. Since there are only 6 bonds attached to it and no lone pairs, the bond angles tend to be 90 degrees, meaning the bonds won't bend.
What causes the bond angles to be 90 degrees?
The top lone pair of electrons repel with the electrons of the surrounding atoms as well as the bottom lone pair. This repulsion at the top and at the bottom balances the bending of the bonds therefore causing the bonds to not bend. This causes the bond angles to be 90 degrees
What contributes to the shape of the central atom?
The only things that contributes to this shape are the 5 bonds which the central atom is attached to and a lone pair of electron. The lone pair of electrons repel with the other atoms electrons surrounding the central atom. This repulsion bends the bonds providing an angle of less than 90 degrees and 90 degrees.
What is a square planar shape?
The Square planar shape is a type of shape which a molecule takes form of when there are 4 bonds attached to a central atom along with 2 lone pairs. If you were to remove 2 bonds from an Octahedral molecule and 1 bond from a Square Pyramidal molecule, it would form a square planar shape. The angle between the bonds is 90 degrees. The shape is non-polar since it is symmetrical. Here are some examples of Square planar-shaped molecules:
What is the shape of a molecule?
The square pyramidal shape is basically an Octahedral shape with 1 less bond. The angle between the bonds is 90 degrees and 84.8 degrees. The shape is polar since it is asymmterical. Here are some examples of Square Pyramidal-shaped molecules:
What is the octahedral shape?
The Octahedral shape is a type of shape which a molecule takes form of when there are 6 bonds attached to a central atom with 4 on the same plane. There are no lone pairs attached to it. The bond angle between the bonds is exactly 90 degrees. The molecule is non-polar since it is a symmetrical shape. Here are some examples of Octahedral-shaped molecules:
Can you add videos to your watch history?
Videos you watch may be added to the TV's watch history and influence TV recommendations. To avoid this, cancel and sign in to YouTube on your computer.
Why do polar molecules have polar bonds?
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. A polar molecule with two or more polar bonds must have an asymmetric geometry so that the bond dipoles do not cancel each other.
What is the net dipole of a polar molecule?
A polar molecule has a net dipole as a result of the opposing charges (i.e. having partial positive and partial negative charges) from polar bonds arranged asymmetrically. (Wikipedia)