Why is photosynthesis considered as endothermic?
Photosynthesis is considered to be an “endothermic reaction” because it absorbs the sun's energy to enable the reaction to happen. Like most of the endothermic reactions, the plants do not absorb heat but they absorb sun's photothermal energy to perform 'photosynthesis' in order to produce starch.
Are photolysis reactions considered exothermic or endothermic?
The reaction between acid and water is an exothermic reaction. This reaction produces a lot of heat and energy which causes the resulting solution to splash. Since the acid is usually more dense than the water adding water to the acid causes the reaction to happen in a small area and on the surface.
How do you know if its endothermic or exothermic?
How do you know if its endothermic or exothermic? An endothermic reaction soaks up heat. An exothermic reaction releases heat. So if the sum of the enthalpies of the reactants is greater than the products, the reaction will be exothermic. If the products side has a larger enthalpy, the reaction is endothermic. Lot more interesting detail can be ...
Which has less pressure exothermic and endothermic?
Thus any transition from a more ordered to a less ordered state (solid to liquid, liquid to gas, or solid to gas) requires an input of energy; it is endothermic. Conversely, any transition from a less ordered to a more ordered state (liquid to solid, gas to liquid, or gas to solid) releases energy; it is exothermic.

Is photosynthesis an example of endothermic reaction?
Photosynthesis requires energy, making it an endothermic reaction. Light, generally sunlight, is the source of this energy. The process converts the sun's electromagnetic energy into chemical energy, which is then stored in chemical bonds in the plant.
Why is photosynthesis an exothermic reaction?
In photosynthesis, energy from light is stored in a high-energy product, glucose. An example of an exothermic reaction is cellular respiration. High-energy glucose releases energy as it's broken down. And that energy is used to generate ATP molecules.
Is photosynthesis and respiration exothermic or endothermic?
As glucose is broken down into carbon dioxide, water, and ATP energy, the respiration reaction is exothermic. The photosynthesis reaction is endothermic because in the presence of sunlight and chlorophyll, carbon dioxide and water, or we can assume the heat is transformed into endothermic glucose reaction.
Why is photosynthesis considered endothermic?
Photosynthesis is considered an endothermic reaction, because during the process of photosynthesis, energy from the sun or sunlight is being absorbed. Any chemical reactions that absorb heat energy from the surroundings to form products is termed the endothermic reaction.
What is the reaction of photosynthesis?
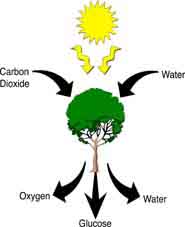
6CO2 + 6H2O + Light C6H12O6 + 6O2 Algae, plants and photosynthetic bacteria take in light through the sun, water molecules from their environment and carbon dioxide from the air and combine these reactants to produce the food i.e. glucose.
Which type of process is photosynthesis?
In chemical terms, photosynthesis is a light-energized oxidation–reduction process.
Does photosynthesis release heat?
In photosynthesis, solar energy is harvested as chemical energy in a process that converts water and carbon dioxide to glucose. Oxygen is released as a byproduct. In cellular respiration, oxygen is used to break down glucose, releasing chemical energy and heat in the process.
What is the heat of reaction of photosynthesis?
Photosynthesis is endothermic, and the necessary energy is supplied by the absorption of solar radiant energy. This energy can be released by carrying out the reverse of Equation 15.13. 1, a process which is exothermic. When we burn paper, wood, or dried leaves, the heat given off is really a stored form of sunlight.
Why is photosynthesis an endothermic reaction?
Photosynthesis is an endothermic reaction because plants absorb energy from the sun, which helps in the production of oxygen through light-dependent reactions.
What is the difference between an exothermic and an endothermic reaction?
Continue Reading. An exothermic reaction is a chemical reaction that releases energy by light or heat. It is the opposite of an endothermic reaction. Expressed in a chemical reaction : reactants → products + energy.
Why won't photosynthesis run in reverse?
But it won’t normally run the other way around because photosynthesis is actually a complex series of reactions, [ 2] each of which would have to operate in reverse. These reactions include the Calvin cycle and the synthesis of carbohydrates. If just one of those steps fails to run in reverse, the whole thing won’t reverse.
How does photosynthesis produce light?
But there’s another way to look at this. Photosynthesis takes light and uses it to create sugars. The logical reverse is to take energy in the form of sugar, metabolize it through glycolysis and then the Krebs cycle to generate ATP, which you can then use to produce light. Fireflies do that using a substance called Luciferin. [ 3]
What is endothermic process?
“endo-” means within or into, while “therm” means heat or energy. So an endothermic process is one in which heat (or energy) is going into the system. Clearly that’s the case with boiling: take a pot of water and set it on the counter.
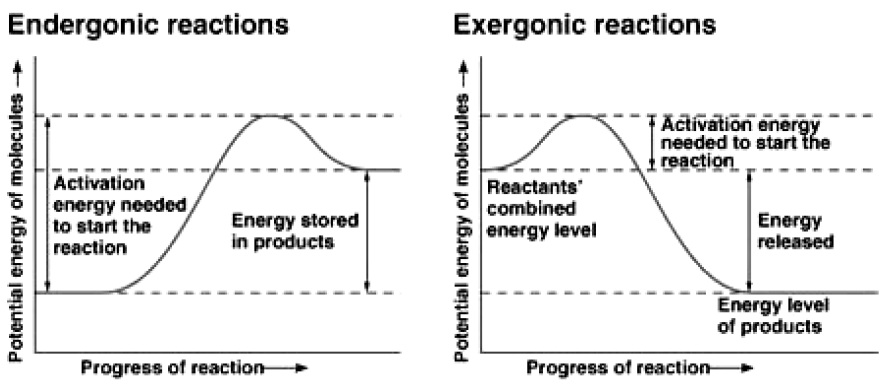
What is the energy profile of an exothermic reaction?
An energy profile of an exothermic reaction. When the medium in which the reaction is taking place gains heat, the reaction is exothermic. When using a calorimeter, the total amount of heat that flows into (or through) the calorimeter is the negative of the net change in energy of the system.
What is a reversible reaction?
A reversible reaction is a reaction where the reactants and products react together to give the reactants back.
What determines whether a reaction is endothermic or exothermic?
What determines whether a reaction is endothermic or exothermic is the balance between the energy that must be supplied to break existing bonds and the energy that is released when new bonds are formed.
What is an exothermic reaction?
In contrast, exothermic reactions are reactions that release energy into the environment in the form of heat. These feel warm or hot, and can even cause an explosion.
Why does energy transfer from the environment to the chemicals that react?
This happens because the energy required to break existing bonds is greater than the energy released when new bonds are formed. In this way, global energy is transferred from the environment to the chemicals that react, absorbing heat.
Is endothermic reaction more common than exothermic reaction?
In this sense, endothermic reactions are less common than exothermic ones, but there are a number that are quite well known.
Is photoynthei endothermic or endothermic?
Photoynthei i a endothermic reaction ince energy in the form of unlight i aborbed by plant. Preciely, in an endothermic reaction, energy i aborbed from the environment. During photoynthei, the pigment
Is thermal decomposition endothermic or endothermic?
Also, any thermal decomposition reaction is endothermic, because the reaction only takes place if heat is introduced into the system. A clear example of this is the degradation of calcium carbonate into calcium oxide and carbon dioxide.
Is photosynthesis an endothermic reaction?
Photosynthesis is a endothermic reaction since energy in the form of sunlight is absorbed by plants. Precisely, in an endothermic reaction, energy is absorbed from the environment.
Content
Photosynthesis and Other Examples of An Endothermic Reaction
- Chemical reactions transfer energy to, or from, the environment. Endothermic reactions absorb energy from the environment, while exothermic reactions transmit energy to the environment. What determines whether a reaction is endothermic or exothermic is the balance between the energy that must be supplied to break existing bonds and the energy that ...
Other Examples of Endothermic Reaction
- -The reaction of crystals from barium hydroxide octahydratewith dry ammonium chloride. -Evaporation of water (water in a liquid state is a compound, and heat is absorbed by breaking the bonds in water molecules). -Dissolution of ammonium chloride in water. -Electrolysis process (the molecules decompose into ions due to the passage of electric current). -The reaction of thionyl …
References
- Exothermic vs. Endothermic and K. (2017 March, 08). In Free Texts. Retrieved on October 2, 2017, from chem.libretexts.org.
- Hall, D. O. and Rao, K. K. (1999). Photosynthesis. New York: Cambridge University Press.
- Helmenstine, A. (2016, March 09). Exothermic Reactions - Definition and Examples. Retrieved on October 02, 2017, from sciencenotes.org.
- Exothermic vs. Endothermic and K. (2017 March, 08). In Free Texts. Retrieved on October 2, 2017, from chem.libretexts.org.
- Hall, D. O. and Rao, K. K. (1999). Photosynthesis. New York: Cambridge University Press.
- Helmenstine, A. (2016, March 09). Exothermic Reactions - Definition and Examples. Retrieved on October 02, 2017, from sciencenotes.org.
- Energy changes in reactions (s / f). On BBC GCSE Bitesize. Retrieved on October 2, 2017, from bbc.co.uk.