.PNG)
How to calculate electronegativity?
Pauling electronegativity given Mulliken electronegativity Solution
- Convert Input (s) to Base Unit
- Evaluate Formula
- Convert Result to Output's Unit
What is electronegativity and how does it work?
Electronegativity is the property of an atom which increases with its tendency to attract the electrons of a bond. If two bonded atoms have the same electronegativity values as each other, they share electrons equally in a covalent bond. Usually, the electrons in a chemical bond are more attracted to one atom (the more electronegative one) than to the other.
What is the formula for electronegativity?
When you're using kJ/mol as units for your energies, the equation for Mulliken electronegativity is ENMulliken = (1.97×10−3) (Ei+Eea) + 0.19. Plug your values into the equation and solve for EN Mulliken . EN Mulliken = (1.97×10 −3 ) (E i +E ea) + 0.19
How to determine electronegative?
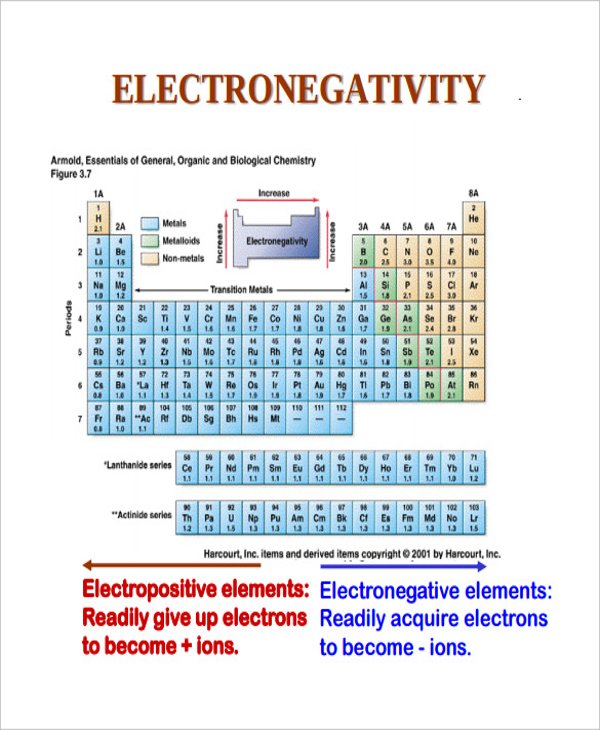
Electronegativity is a measure of an atom's ability to attract shared electrons to itself. On the periodic table, electronegativity generally increases as you move from left to right across a period and decreases as you move down a group. As a result, the most electronegative elements are found on the top right of the periodic table, while the least electronegative elements are found on the bottom left.
How does polarity relate to electronegativity?
Electrons in a polar covalent bond are shifted toward the more electronegative atom; thus, the more electronegative atom is the one with the partial negative charge. The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms.
Does polarity increase with electronegativity?
Bond polarity and ionic character increase with an increasing difference in electronegativity. As with bond energies, the electronegativity of an atom depends to some extent on its chemical environment.
What determines polarity?
The polarity of a bond is determined by a periodic concept called electronegativity. Electronegativity is an expression of an atom's tendency to attract electrons in a chemical bond. In order to determine the polarity of a bond, you must find the difference in the electronegativies of the atoms involved.
How do you determine polarity without electronegativity?
To review the steps:Draw the Lewis structure.Figure out the geometry (using VSEPR theory)Visualize or draw the geometry.Find the net dipole moment (you don't have to actually do calculations if you can visualize it)If the net dipole moment is zero, it is non-polar. Otherwise, it is polar.
What affects polarity?
The shape of a molecule and the polarity of its bonds determine the OVERALL POLARITY of that molecule.
How does the electronegativity difference of the atoms affect bond polarity?
Electrons in a polar covalent bond are shifted toward the more electronegative atom; thus, the more electronegative atom is the one with the partial negative charge. The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms.
How do you determine the polarity of a bond?
You'll need to take the difference between the electronegativity value of the two atom. Their bond polarity is determined according to the range it falls in: Nonpolar covalent: Electronegativity difference < 0.5. Polar covalent: Electronegativity difference is 0.5 - 1.6.
What causes bond polarity?
The polarity of bonds is caused due to the interaction of the bonds between molecules and atoms with different electronegativities. Consider an electromotive force (EMF) or an electric potential, acting between two points. Here, the points or poles have a greater number of electrons than the other.
Electronegativity Property of Atoms
Electronegativity expresses an element’s ability to attract electrons. This attraction comes from the positive charge within the nucleus.
Electronegativity From
The basis of electronegativity comes from two opposing factors: the distance of valence electrons from a positive nuclear charge and the size of the positive nuclear charge, Figure 2.
In A Bond
When two different elements share a bond, a state of polarity exists between them. A polar bond means the covalent electrons between the atoms are not equally shared between atoms.
Polarity
To show ta polar bond between two elements, an arrow indicates the direction of a partial charge. The arrow points towards more electron density.
What is polar bond?
I have spoken about electronegativity and mentioned polar bonds. To be precise a polar bond is a bond with an uneven distribution of charge which means that if the bond has an electronegativity difference then the bond is also polar.
Why are the carbon and chlorine bonds polar?
The carbon – chlorine bonds are all polar because of the electronegativity difference between the two atoms BUT this is NOT a polar molecule. Because all of the four bonds are polar there is now not an uneven distribution of charge across the molecule, and it is therefore not polar.
How many electrons does fluorine have?
Both carbon and fluorine are in period two, so this means they both have a full inner orbital with two electrons. The carbon atom has six protons in the nucleus and the fluorine atoms has nine.
What is the measure of the ability of an atom to attract a bonding pair of electrons?
Electronegativity . Electronegativity is a measure of the ability of an atom to attract a bonding pair of electrons and the values usually given from the Pauling scale (for A-level anyways).
Is fluorine polar or nonpolar?
As you can see this means that the electron pair in the covalent bond is (on average) much closer to the fluorine atom compared to the carbon atom. This is a polar bond.