What is the function of the prothrombinase complex?
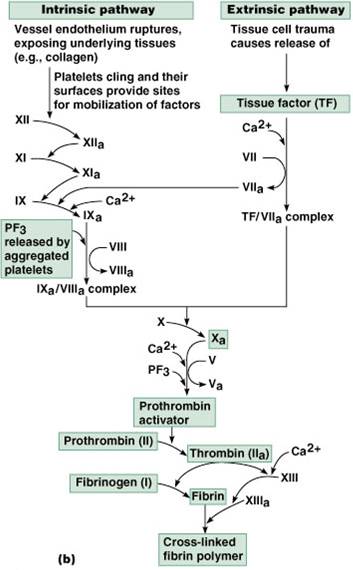
This enzyme complex, the prothrombinase complex which consists of the serine protease factor Xa (FXa) and cofactor factor Va (FVa) bound to an anionic membrane surface, plays an important role in blood coagulation as it rapidly converts prothrombin to thrombin, the latter being a key regulatory enzyme in the formation of a blood clot [6].
What is the substrate of prothrombinase?
The substrate, prothrombin, is a circulating single-chain vitamin K–dependent zymogen produced by the liver. Within the prothrombinase complex, FXa cleaves prothrombin at 2 sites producing a two-chain (A and B) active protease, called α-thrombin, held together by a single disulfide bond.
What enzyme converts prothrombin to the mature enzyme at R320?
The prothrombinase complex converts prothrombin to the mature enzyme along two pathways by cleaving sequentially at R271 and R320 (prothrombin numbering).
What is the difference between factor Xa and prothrombinase?
Prothrombinase. The prothrombinase complex can catalyze the activation of prothrombin at a rate 3 x 10 5 -fold faster than can Factor Xa alone. Thus, the prothrombinase complex is required for the efficient production of activated thrombin and also for adequate hemostasis.
What type of enzyme is prothrombinase?
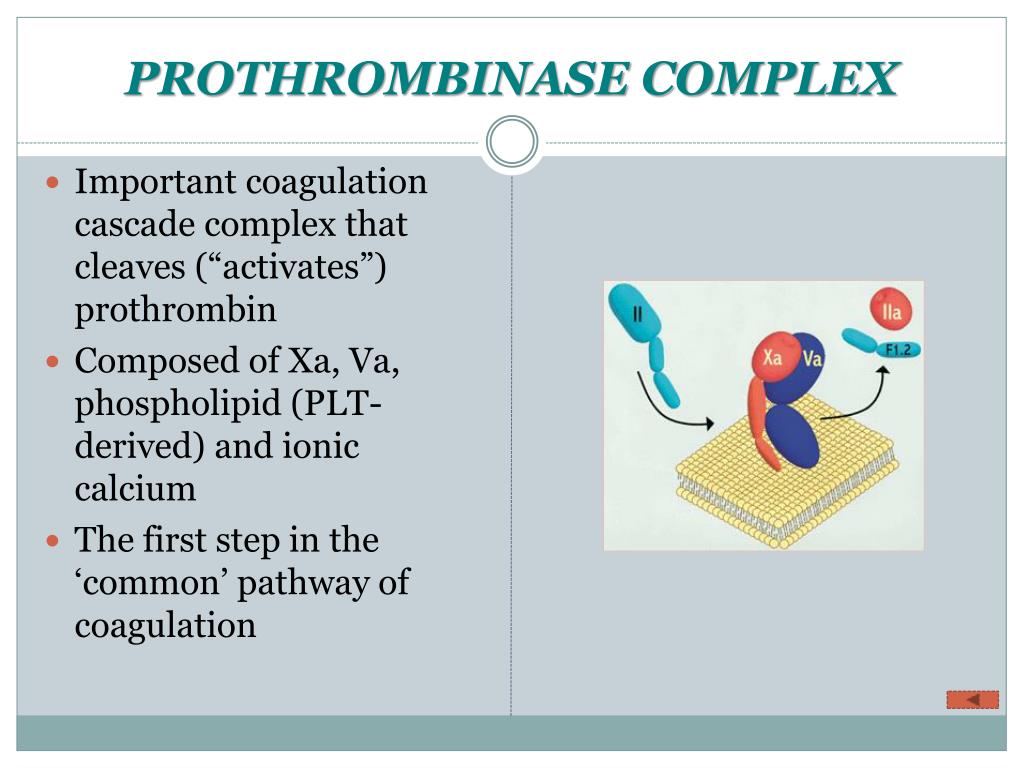
Being the only physiologic producer of thrombin, the prothrombinase complex is essential for hemostasis. Factor Xa binds to factor Va, its activated cofactor, on anionic phospholipid membrane surfaces to form the prothrombinase complex.
Is prothrombin a protein or enzyme?
Prothrombin is a protein of molecular mass about 68,000. It is an α-globulin and present in the plasma at a concentration of 300 Iowa units/ml or 15 μg/ml. It is converted to the active enzyme thrombin by the action of several factors known as plasma or tissue thromboplastins (Fig. 1).
What is prothrombinase made of?
The prothrombinase complex consists of the serine protease, Factor Xa, and the protein cofactor, Factor Va. The complex assembles on negatively charged phospholipid membranes in the presence of calcium ions.
Is prothrombin an enzyme or a hormone?
Answer: all these are enzymes helping inblood clotting .
Is fibrinogen an enzyme?
Fibrinogen is the major clotting protein of plasma, which upon cleavage by thrombin is converted into fibrin. In the present investigation, SufA, a subtilisin-like enzyme of F. magna, was found to modulate this target protein of the coagulation cascade and efficiently inhibit the formation of a fibrin network.
Is thromboplastin an enzyme?
Thromboplastin (TPL) is derived from cell membranes and is a mixture of both phospholipids and tissue factor, neither of which are enzymes.
What is the function of prothrombinase?
The prothrombinase complex plays a pivotal role in the coagulation cascade. It is responsible for the proteolytic conversion of prothrombin to thrombin-which in turn is involved directly in the formation of fibrin, activation of platelets, and feedback activation of other components of the cascade.
What is another name for prothrombinase?
Prothrombin is transformed into thrombin by a clotting factor known as factor X or prothrombinase; thrombin then acts to transform fibrinogen, also present in plasma, into fibrin, which, in combination with platelets from the blood, forms a clot (a process called coagulation).
How is prothrombinase produced?
The propagation phase of coagulation is promoted by the incorporation of calcium-mediated binding of factor Xa to the platelet surface with factor Va, creating the prothrombinase complex. In this complex, large amounts of prothrombin can be converted to thrombin to promote coagulation.
Is Thrombokinase and prothrombinase same?
Answer: Answer: prothrombinase is (biochemistry) a complex consisting that catalyzes the conversion of prothrombin to thrombin in the presence of calcium ions while thrombokinase is (enzyme) a proteolytic enzyme, that converts prothrombin into thrombin during the clotting of blood.
What enzyme converts thrombin to prothrombin?
The proteolytic conversion of prothrombin to thrombin catalyzed by prothrombinase is one of the more extensively studied reactions of blood coagulation. Sophisticated biophysical and biochemical insights into the players of this reaction were developed in the early days of the field.
What enzymes cause blood clots?
Blood-clotting proteins generate thrombin, an enzyme that converts fibrinogen to fibrin, and a reaction that leads to the formation of a fibrin clot.
Is prothrombin a protein?
Prothrombin (also called coagulation factor II) is one of the key proteins in the blood coagulation system. After enzymatic cleavage, prothrombin is converted to the active form – thrombin (factor IIa), catalyzing the conversion of fibrinogen to fibrin, thus ensuring clot formation.
Is fibrinogen a protein?
Fibrinogen and fibrin are multifunctional proteins. Fibrinogen is indispensable for platelet aggregation; it also binds to several plasma proteins, however, the biological function of this interaction is not completely understood.
What is difference between thrombin and prothrombin?
The key difference between thrombin and prothrombin is that thrombin is an enzyme that facilitates blood clotting by converting fibrinogen to fibrin, while prothrombin is a glycoprotein that is converted into thrombin during bleeding and subsequent clotting.
Where does prothrombin come from?
Prothrombin, the inactive precursor to thrombin, is synthesized by the liver in a vitamin K-dependent reaction and is released into the circulation.
What is the process of forming thrombin?
The generation of thrombin requires the formation of the prothrombinase complex, which consists of FXa, phospholipid, calcium, and a protein cofactor, FV (Gentry, 2004) (see Fig. 10-2 ). The complex catalyzes two cleavages in prothrombin, at Arg320 (to produce meizothrombin) and at Arg271, leading to the formation of thrombin ( Autin et al., 2006 ). The first few molecules of thrombin generated by this prothrombinase complex initiate several positive-feedback reactions that sustain its own formation and facilitate the rapid growth of the blood clot or thrombus around the area of vascular damage. For example, thrombin can convert FXI to its proteolytically active form, FXIa, which, in turn, converts FIX to FIXa. The thrombin-induced conversion of FV to FVa, along with the increased availability of FXa, greatly enhances the rate and extent of thrombin formation by the prothrombinase complex. This is a crucial reaction for normal blood coagulation. FXa alone can slowly activate PT, but the rate of thrombin formation is enhanced several orders of magnitude by the presence of FVa in the PTase complex ( Autin et al., 2006 ). Another positive feedback response is the increased availability of phospholipids on the surface on thrombin-activated platelets that accumulate at sites of vascular damage ( Gentry, 2004 ).
What is the role of factor XA in hemostasis?
Being the only physiologic producer of thrombin, the prothrombinase complex is essential for hemostasis. Factor Xa binds to factor Va, its activated cofactor, on anionic phospholipid membrane surfaces to form the prothrombinase complex. Activated platelets release factor V from their α-granules, and this platelet-derived factor V may play a more important role in hemostasis than its plasma counterpart.17 Whereas plasma factor V requires thrombin activation to exert its cofactor activity, the partially activated factor V released from platelets already exhibits substantial cofactor activity. Activated platelets express specific factor Va binding sites on their surface, and bound factor Va serves as a receptor for factor Xa. The catalytic efficiency of factor Xa activation of prothrombin increases by 10 5 -fold when factor Xa incorporates into the prothrombinase complex. 13 Prothrombin binds to the prothrombinase complex, where it undergoes conversion to thrombin in a reaction that releases prothrombin fragment 1.2 (F1.2). Plasma levels of F1.2, therefore, provide a marker of prothrombin activation. Prothrombin is the most abundant coagulation factor, and the efficiency of activation generates high local levels of thrombin.
What residues are in the B chain?
The B chain contains all residues responsible for catalytic activity, substrate recognition, and allosteric regulation. Trypsin-like specificity for Arg residues at P1 54 is conferred to thrombin by the presence of D189 in the S1 site occupying the bottom of the catalytic pocket. Unlike trypsin, however, thrombin can efficiently cleave chymotrypsin-specific substrates carrying Phe at the P1 position, 55 as documented more than 40 years ago from studies on ester substrates. 56–58 Thrombin has a preference for small and hydrophobic side chains at P2 that pack tightly against the hydrophobic wall of the S2 site defined by residues Y60a-P60b-P60c-W60d of the 60-loop. Residues at P3 point away from the thrombin surface, whereas aromatic and hydrophobic residues at P4 tend to fold back on the thrombin surface 59 and engage the aryl binding site defined by L99, I174, and W215. 44 An important exception has been identified recently from the structure of thrombin in complex with the uncleaved extracellular fragment of PAR1, where the acidic residue at the P3 position of substrate makes an H-bonding interaction with the backbone N atom of G219 instead of pointing away from the thrombin surface. 60 The autolysis loop shapes the lower rim of access to the active site and contributes to recognition of fibrinogen 61 and the intrinsic allosteric properties of the enzyme. 39 The loop centered on K70 defines exosite I and is homologous to the Ca 2+ binding loop of trypsin and chymotrypsin. 62 In these proteases, Ca 2+ stabilizes the fold and confers increased resistance to proteolytic digestion. In thrombin, the need for Ca 2+ is eliminated by insertion of K70 in the cavity available for binding this cation. Thrombin does not bind Ca 2+ up to mM concentrations. 23,63 Exosite I contains several positively charged residues that give rise to an intense electrostatic field. The field provides steering and optimal preorientation for fibrinogen, thrombomodulin, the natural inhibitor, hirudin, and PAR1 to facilitate formation of a productive complex upon binding. Structural and site-directed mutagenesis data support exosite I as a binding epitope for fibrinogen, 22,64,65 fibrin, 22,66 thrombomodulin, 64,67–70 and the thrombin receptors, PAR1 22,60,71,72 and PAR3. 22,73 On the side of the enzyme opposite to exosite I, a C-terminal helix and its neighbor domains host a number of positively charged residues and define exosite II. This site is the locale for interaction with polyanionic ligands, like heparin and glucosaminoglycans, 74–78 and fragment 2 in thrombin precursors. 79,80 Heparin enhances inhibition of thrombin by antithrombin via a template mechanism in which a high affinity heparin–antithrombin complex is first formed and then docked into exosite II and the thrombin active site by electrostatic coupling. 77,81–83 Exosite II is also the locale for thrombin interaction with the platelet receptor GpIb, 84–87 the acidic moiety of the fibrinogen γ′ chain, 88 and has been involved in the binding of autoantibodies. 89
How do activated platelets protect the plasma-derived FVA from inactivation by APC?
Activated platelets also protect the normal plasma-derived FVa from inactivation by APC by slowing the cleavage at Arg 506. 104 However, in contrast to the platelet-derived cofactor, plasma-derived FVa can be completely inactivated by APC, suggesting that the two different cofactor pools (plasma vs. platelet) are different substrates for APC. 104 These studies again confirm the importance of binding events in modulating prothrombinase activity. They also demonstrate that platelet and synthetic phospholipid membranes are not equivalent surfaces in regulating enzyme complex formation. Collectively, these studies indicate that platelets sustain procoagulant events by providing a membrane surface that delays cofactor inactivation and by releasing a cofactor molecule that displays an APC-resistant phenotype.
What is the prothrombinase complex?
The prothrombinase complex converts prothrombin to the mature enzyme along two pathways by cleaving sequentially at R271 and R320 (prothrombin numbering). Initial cleavage at R320 (R15 in the chrymotrypsin numbering) between the A and B chains is the preferred pathway under physiological conditions and generates the active intermediate, meizothrombin, by triggering formation of the I16–D194 ion-pair, and structuring of the active site and oxyanion hole.43 The alternative initial cleavage at R271 sheds the Gla domain and the two kringles and generates the inactive precursor, prethrombin-2, with the R15–I16 peptide bond intact. Thrombin is composed of two polypeptide chains of 36 (A chain) and 259 (B chain) residues that are covalently linked through a disulfide bond between residues C1 and C122. 17,44 The standard orientation 44 puts the A chain at the back of the molecule, opposite to the front hemisphere of the B chain that hosts the entrance to the active site and all known functional epitopes of the enzyme ( Fig. 1 ). 20 The A chain has received little attention in thrombin studies and is considered an appendage of the activation process from prothrombin. Previous studies have suggested that the A chain may be dispensable for function. 45,46 However, several naturally occurring mutations of prothrombin involve residues of the A chain 47–50 and are associated with severe bleeding. The functional defects in prothrombins Denver (E8K and E14cK), 47 Segovia (G14mR), 49 and San Antonio (R15H) 50 have been attributed to perturbation of the zymogen → enzyme conversion and processing by factor Xa, resulting in severe bleeding. Such explanation is obvious for the G14mR and R15H mutations that affect the P1 (R15) and P2 (G14m) sites of recognition by factor Xa, but not for the E8K and E14cK mutations of prothrombin Denver. Other naturally occurring mutations, like deletion of K9 or K10, 48 are also associated with severe bleeding. Interestingly, the defect causes impaired fibrinogen and PAR1 cleavage, reduced response to Na + activation, 51,52 and long-range perturbation of active site residues. 52 Recent mutagenesis of the A chain has explained the phenotype observed in naturally occurring mutations and pointed out the importance of this domain in thrombin function. 53
How do platelets help with thrombosis?
Platelets can promote thrombosis by providing surfaces favoring the assembly of prothrombinase, activation of factor XII, and cross-linking of fibrin mediated by factor XIII. Platelets also can inhibit thrombolysis by the release of PAI-1 and α2 -antiplasmin. Inhibition of platelet activation should potentiate clot lysis. At least five pathways exist through which activation of platelets can occur.
Which enzyme is a cofactor-interactive site?
This evidence includes peptide mapping studies, which identified the fXa segment, Val231 to Thr244, as one cofactor-interactive site. Additionally, the fXa segment, Ser241-Lys252, was shown to contribute to the binding of its substrate, that is, prothrombin. 117
What is the role of factor VIIA in coagulation?
During the initiation phase of coagulation, tissue factor–mediated factor VIIa is able to create small amounts of factor Xa, which then converts initial amounts of prothrombin (factor II) to thrombin (factor IIa). This initial creation of thrombin is able to promote its own production through activation of factors V and VIII.
What is the substrate of FXA?
The substrate, prothrombin, is a circulating single-chain vitamin K–dependent zymogen produced by the liver. Within the prothrombinase complex, FXa cleaves prothrombin at 2 sites producing a two-chain (A and B) active protease, called α-thrombin, held together by a single disulfide bond.
How does prothrombinase formation affect catalysis?
Prothrombinase formation defines an important modulating event catalytically, because the complete complex is 300,000 times more efficient than free factor Xa acting on prothrombin in solution.24,72 An implication of this significant rate increase is that any dissociation of components from the complex significantly and drastically decreases enzyme activity. The absence of the membrane surface from the complete catalyst results in a 1000-fold loss in catalytic efficiency, whereas deletion of factor Va from the complex results in a 10,000-fold decrease in the rate of thrombin generation. 24 The membrane surface is obligately required because, although contributing little to the intrinsic rate of catalysis, it provides an environment in which both the factor Va–factor Xa complex and substrate can coconcentrate. 277 Prothrombin appears to concentrate at the platelet membrane surface in large part through the receptor-mediated mechanisms detailed earlier. Factor Va, by virtue of its membrane binding capacity, forms an obligate and high-affinity interaction with factor Xa, because factor Xa will not bind to activated platelets in the absence of factor Va. Concentration of reactants at the membrane surface results in high local concentrations of reagents that exceed the intrinsic K m of the reaction and significantly improve catalytic efficiency. In addition, factor Va enhances the turnover rate of the reaction by a mechanism that has not yet been rationalized. 278
What is the function of FXA?
FXa activation represents the merging point of the intrinsic and extrinsic pathways ( Figure 1 ). FXa activates prothrombin to thrombin , the final clotting protease responsible for the conversion of soluble fibrinogen to insoluble fibrin, leading to blood clotting. Thrombin activation occurs on the surface of activated platelets and requires formation of a prothrombinase complex composed of the platelet phospholipids, Ca 2+, FVa cofactor and FXa ( Monroe et al., 2002 ). Similar to FVIIIa in the tenase complex, FVa is a potent enhancer in the prothrombinase complex. FVa acts as a receptor for FXa and binds in a Ca 2+ -dependent manner to PS-rich surfaces of activated platelets where it forms a complex with FXa and the substrate prothrombin. FVa strongly enhances the catalytic efficiency of prothrombinase complex, probably by concentrating and properly orienting the enzyme (FXa) and the substrate (prothrombin) in the complex ( Mann et al., 1981 ). A gain of function mutation in FV (FV Leiden) causes the most common hypercoagulability disorder in humans ( Bertina et al., 1994 ).
What is the role of factor X in blood clotting?
This pathway is called the ‘extrinsic pathway’ and is responsible for the initiation of coagulation, proceeding mainly on the surface of damaged endothelial cells and macrophages, but probably also on activated platelets [58,59]. Alternatively, factor X is activated on the platelet surface by a membrane-bound ‘tenase’ complex comprising factor IXa, its cofactor factor VIIIa, and calcium ions, which activates factor X ~ 10 6 -fold more rapidly than factor IXa alone [5]. This ‘intrinsic pathway’ is responsible for amplifying the coagulation process (see also Chapter 640) and its importance is illustrated by the fact that hereditary deficiency of factors IX or VIII causes hemophilia B and A, respectively. Thus, factor X plays a pivotal role in blood clotting at the point of convergence of the two coagulation pathways . Accordingly, several rare mutations in the factor X gene have been identified that give rise to bleeding tendencies of variable severity ( e.g. Chafa et al. [60], Bereczky et al. [61] ). Theoretically, injection of factor Xa into patients with hemophilia should bypass the intrinsic pathway and permit generation of thrombin, but this fails because of the short half-life in plasma of factor Xa. However, mutants in which Ile16 or Val17 are replaced have a much longer half-life because they do not form complexes with antithrombin III or tissue factor inhibitor in hemophiliac plasma, yet still are able to activate prothrombin and thus may be useful therapeutic agents [62,63].
How do activated platelets protect plasma derived factor Va?
Activated platelets also protect the normal plasma-derived factor Va from inactivation by activated protein C by slowing the cleavage at Arg 506. 110 However, in contrast to the platelet-derived cofactor, plasma-derived factor Va can be completely inactivated by activated protein C, suggesting that the two different cofactor pools (plasma vs. platelet) are different substrates for APC. 110 These studies again confirm the importance of binding events in modulating prothrombinase activity. They also demonstrate that platelet and synthetic phospholipid membranes are not equivalent surfaces in regulating enzyme complex formation. Collectively, these studies indicate that platelets sustain procoagulant events by providing a membrane surface that delays cofactor inactivation and by releasing a cofactor molecule that displays an activated protein C-resistant phenotype.
How does intracellular Xase modulate catalytic efficiency?
Intrinsic Xase is modulated by a variety of mechanisms similar to those observed with prothrombinase. Complex formation results in an approximate 2 × 10 7 -fold increase in catalytic efficiency. 217 The binding of factors VIIIa and IXaβ to thrombin-activated platelets decreases the K m for factor X activation 2500-fold and enables factor VIIIa to increase the k cat approximately 7500-fold. Antithrombin III cannot inhibit factor IXaβ in the presence of factor VIIIa and platelets. 279 Similar to factor Va, complex formation of factor VIIIa with factor IXaβ modulates its activity. The dissociation of the A2 subunit from the heterotrimer factor VIIIa, which results in a complete loss of cofactor activity, is prevented by its association with factor IXaβ on a membrane surface, 280 as is its inactivation by APC. 62,281
Abstract
This review is a brief summary of the history of the development of the Prothrombinase complex paradigm and its incorporation into the “extrinsic pathway”. It summarizes my laboratory’s research from 1968 to 2012 and identifies many of the key players in these efforts.
Ethics declarations
This review covers 44 years of research and over 400 publications from my laboratory. I do not have any conflict of interest, nor any of my family members, dealing with this research.
Additional information
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
What is the role of prothrombinase in blood clots?
The macromolecular enzyme complex prothrombinase serves an indispensable role in blood coagulation as it catalyzes the conversion of prothrombin to thrombin , a key regulatory enzyme in the formation of a blood clot. Interestingly, a virtually identical enzyme complex is found in the venom of some Australian elapid snakes, which is composed of a cofactor factor Va-component and a serine protease factor Xa-like subunit. This review will provide an overview of the identification and characterization of the venom prothrombinase complex and will discuss the rationale for its powerful procoagulant nature responsible for the potent hemostatic toxicity of the elapid venom.
What are the groups of prothrombin?
Prothrombin converting enzymes are commonly found in snake venoms and they are classified into four groups (A, B, C, and D) depending on their cofactor requirements in prothrombin activation [7,8,9]. Group A and B prothrombin activators are metalloproteases that convert prothrombin to the active intermediate meizothrombin; they are found in the venom of several vipers [9]. Group C and D prothrombin activators are serine proteases that are capable of fully activating prothrombin to thrombin and are exclusively found in Australian snakes [8,9]. Whereas group D activators require calcium, phospholipids, and the protein cofactor factor V (FV) for optimal protease activity, group C activators function in the absence of this cofactor. It is these group C activators that have, so far, only been observed in the Elapidae family members and have been the subject of detailed studies. These studies have shown that, remarkably, the group C activator complex consists of a FVa-like subunit and a FXa-like subunit similar to the blood coagulation prothrombinase complex [2,4]. To date, this subset of snakes are the only species identified that have two sources of prothrombinase: one that circulates in plasma and is required for normal hemostasis and one that is present in the venom and likely plays an important role in the envenomation of prey.
How does FVa stabilize?
The mammalian FVa structure is stabilized by several interdomain contacts linking the C1-C2, A3-C1, and A3-C2 domains [33]. Metal ion binding further contributes to these interdomain interactions by providing stability to the local structure [33]. Analysis of the A3-C1-C2 residues involved in direct interdomain contacts indicates that all of the residues connecting the C1-C2 and A3-C2 domains are conserved in venom FV, while the hydrophobic interactions linking the A3-C1 domains are at least partially preserved. Of the residues implied in Cu2+binding, the majority are found in venom FV, as well as several residues involved in essential interactions between A1 and A3. Furthermore, the high affinity Ca2+binding site is completely conserved. This implies that heavy and light chains of venom FV are linked in a manner similar to mammalian FV.
What is the B domain of venom?
A remarkable feature of the FV homologs expressed in the elapid venom is that their B domain is extraordinarily short: 46 versus~600–800 residues in mammals (the FV B domain is defined as the region removed following thrombin cleavage to generate the heavy and light chains; Figure 2) [30]. Our laboratory has previously shown that the B domain plays an important function by interfering with functional binding interactions of FV through imposing steric and/or conformational constraints, thereby preventing constitutive cofactor function [31,32]. In addition, we have identified a discrete region of the B domain that plays a critical role in stabilizing the procofactor state [32]. Part of this region (residues 963–1008 of human FV) is unusually basic with 18 of 46 residues being Arg or Lys, and is well conserved across the vertebrate lineage [30]. Given that venom FV lacks the majority of the B domain sequence, it is not surprising that the so called basic region is absent as well. This intriguing observation prompted us to assess the cofactor capacity of purified recombinant venom-derived P. textilisFV (pt-FV) [27]. Consistent with our previous observations, we were able to show that the absence of the basic region correlates with the expression of procoagulant FV activity, indicating that venom FV is expressed as a constitutionally active FV variant [27]. As such, this is the first FV species observed thus far that exists as a constitutively active cofactor. Interestingly, liver-expressed P. textilisFV has a similar short B domain that lacks the basic region [19], which would suggest that the FV circulating in the elapid’s plasma is constitutionally active as well; however, biochemical studies are needed to confirm this possibility.
What are the effects of envenomation?
One of the major clinical effects of envenomation by the Australian Elapidae genera Pseudonajaand Oxyuranusis significant consumptive coagulopathy, causing early hypotension, spontaneous bleeding, and severe fibrinogen depletion [37,38,39,40]. It is thought that the injected prothrombin activating complex is responsible for these symptoms, as it rapidly converts the prey’s prothrombin to thrombin, which subsequently leads to the formation of microthrombi. This process can be life threatening in several ways: the microthrombi could get lodged in the microvasculature of the lungs, thereby resulting in a pulmonary embolism, and the formation of microthrombi severely exhausts the blood coagulation system (coagulopathy), giving rise to fibrinogen depletion and spontaneous bleedings.
How much venom does a snake produce?
The amount of venom produced by a snake when it bites (e.g., venom yield) has been found to vary from 8 to 146 mg for the Pseudonajaand Oxyuranussnakes [43]. Considering that the prothrombinase-like complex makes up 5–40% of the yield [2,3,4,5], 0.4–58 mg or 2–260 nmols (FXa-FVa ~220 kD) of the enzyme complex could be injected into the prey. Assuming that the kinetic parameters for mammalian prothrombin conversion are similar to that of human prothrombin, the amount of thrombin generated in 30 minutes can go from 20 µmols up to 2 mmols (kcat~ 300 min−1) [27]. This exceeds the amount of thrombin required for clot formation at the site of injury by several orders of magnitude, which is generally assumed to be in the nanomolar range [44]. Based on these values, it is not surprising that the first symptoms of consumptive coagulopathy can be observed within half an hour following envenomation [38,40].
What are the posttranslational modifications of factor V?
Factor V undergoes multiple posttranslational modifications, including N-glycosylation, phosphorylation, and sulfation at multiple sites [29], which are not fully conserved in venom FV. Mammalian FV is heavily N-glycosylated, and 25 out of the 37 putative N-glycosylation sites are located within the B domain (Figure 1). Venom FV, on the other hand, has 16 potential N-glycosylation sites of which one is in the B domain (Figure 1) [34]. Some of these N-glysocylation sites are similar to human FV, whereas others are unique to snake FV. Interestingly, liver-expressed P. textilisFV has an additional putative N-glycosylation site in the B domain at Asn573[19], which is not preserved in any of the venom FV species. Phosphorylation of the human FVa heavy chain at Ser692has been implied to enhance the rate of APC-dependent inactivation [35]. Although this phosphorylation site is absent in the venom FVa-like subunit, venom-derived P. textilisFV could be proteolyzed by human APC as discussed above [27]. This suggests that phosphorylation of the FVa heavy chain may not be critical to inactivation by APC per se. Sulfation of FV has been speculated to be of importance for the recognition of FV by thrombin and for full FVa cofactor activity [36]. However, out of the six sulfation sites present in human FV, the one homologous to human Tyr1593is conserved in snake venom FV. Furthermore, our data on the thrombin-mediated pt-FV activation indicate a minor role for sulfation in the recognition of venom FV by thrombin [27].