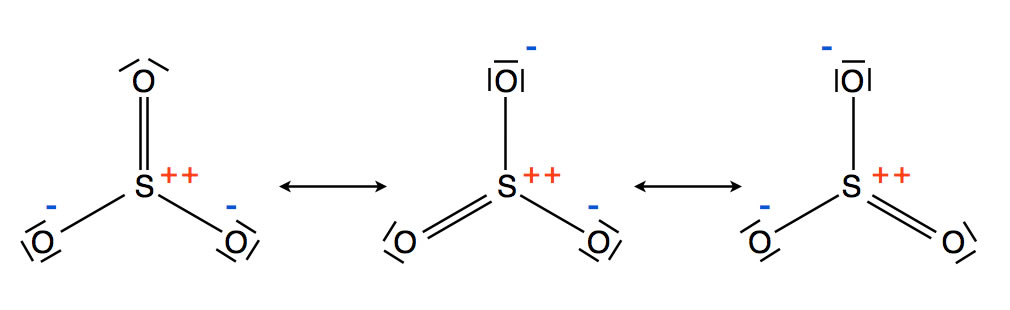
How many valence electrons are in SO3?
Valence: Here, sulfur in the center because of its lowest electron capability, and three oxygen around it. Sulfur brings 6, and oxygen brings 3 each. That means; SO3 has 24 valence electrons. 6 + (3 x 6) = 24. When we draw it, firstly we get the three structures at the top.
What are the side effects of using organic sulfur?
What Are the Side Effects of Using Organic Sulfur? Methylsulfonylmethane, sometimes referred to as organic sulfur, can cause nausea, diarrhea and headaches, according to Drugs.com. Some individuals experience severe allergic reactions to organic sulfur. Symptoms of an allergic reaction include hives, itching, swelling and difficulty breathing.
Is SO3 a solid?
Sulfur trioxide is a solid at just below room temperature with a relatively narrow liquid range. Gaseous SO 3 is the primary precursor to acid rain. The molecule SO 3 is trigonal planar. As predicted by VSEPR theory, its structure belongs to the D 3h point group.
What are the side effects of sulfur dioxide?
… Call your doctor at once if you have:
- severe burning, redness, or swelling where the medicine was applied;
- severe dryness or peeling of treated skin; or.
- new or worsening skin symptoms.

What is SO3 classified as?
Sulfur trioxide (alternative spelling sulphur trioxide, also known as nisso sulfan) is the chemical compound with the formula SO3. It has been described as "unquestionably the most important economically" sulfur oxide.
Are SO2 and SO3 gases?
SO2 and SO3 are inorganic chemical compounds formed by the combination of sulfur atoms and oxygen atoms. SO2 stands for sulfur dioxide, and SO3 stands for sulfur trioxide. These are gaseous compounds.
Is SO2 a gas?
Sulfur dioxide, SO2, is a colorless gas or liquid with a strong, choking odor. It is produced from the burning of fossil fuels (coal and oil) and the smelting of mineral ores (aluminum, copper, zinc, lead, and iron) that contain sulfur.
How do you make SO3 gas?
Sulfur trioxide is formed by reacting sulfur dioxide with oxygen in the present of platinum catalyst. 40 cm long, hard melted glass tube is connected to the ice-cooled receiver. In the tube 10 cm platinized asbestos is placed.
Why is SO2 gas and SO3 solid?
The key difference between SO2 and SO3 is that SO2 is a colourless gas at room temperature, whereas SO3 is a colourless to white crystalline solid. SO2 is sulfur dioxide while SO3 is sulfur trioxide. Both are oxides of sulfur.
How do you test for SO3 gas?
Allow a drop of water to fall on one of the crystals; the chemical combination is so intense that the water hisses as it does when falling on red-hot iron. If you test this solution you will find it is sulphuric acid.
How do you make SO2 gas?
0:020:57Laboratory Preparation of Sulphur Dioxide - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe flask is gently heated reaction takes place and sulphur dioxide is evolved in a continuousMoreThe flask is gently heated reaction takes place and sulphur dioxide is evolved in a continuous stream. Also dioxide being heavier than air is collected in a gas jar by upward displacement of. Air you.
Why is SO2 a gas at room temperature?
Thus, sulfur dioxide exists as a gas at room temperature because its intermolecular forces are not strong enough for the molecules to be dense enough for anything other than gas to form at room temperature.
Is so3 poisonous?
It is corrosive to metals and tissue. It causes eye and skin burns. Ingestion causes severe burns of mouth esophagus and stomach. The vapor is very toxic by inhalation.
What does SO3 smell like?
It is a colorless gas that smells like burnt matches. Sulfur trioxide is often a colorless or white solid that creates white fumes in the air and has strong reactions with water. Both sulfur dioxide and sulfur trioxide react to form sulfuric acid, which is toxic to living tissue and is the main component of acid rain.
What is structure of SO3?
The structure of sulfur trioxide is trigonal pyramidal, as the double bond is formed there are no lone pair exists on the central atom sulfur. Sulfur trioxide does not have a charge and the oxygen atoms are so electronegative that sulfur surrenders its electron to them, because sulfur is weak compared to oxygen.
Is SO3 soluble in water?
To form an aqueous solution of sulfuric acid (H2SO4), sulphur trioxide (SO3) dissolves in and reacts with water.
Is SO3 a gas at room temperature?
Sulfur trioxide (SO3) is generally a colorless liquid. It can also exist as ice- or fiber-like crystals or as a gas. When SO3 is exposed to air, it rapidly takes up water and gives off white fumes. It can react with water to form sulfuric acid.
What is the difference between SO2 and SO3 and so4?
Answer: These compounds are called oxides of sulfur since they are formed from the reaction between sulfur and O2 molecules. The main difference between SO2 and SO3 is that SO2 has two oxygen atoms bonded to a sulfur atom whereas SO3 has three oxygen atoms bonded to a sulfur atom.
Which is more harmful SO2 or SO3?
SO3 is more harmful air pollutant than SO2.
What type of oxide is SO2?
sulfur oxide, any of several compounds of sulfur and oxygen, the most important of which are sulfur dioxide (SO2) and sulfur trioxide (SO3), both of which are manufactured in huge quantities in intermediate steps of sulfuric acid manufacture.
What is the compression factor of a gas?
Since PV = nRT , for an ideal gas PV/nRT must be equal to 1. We call this the compression factor. Let us look at the compression factor of some real gases.
What is the most ideal gas?
The most “ideal” real gas would be helium owing to its size and inert nature. The next one is probably hydrogen.
Why is iron pirite not directly dissolved in water?
It is not directly dissolved in water because direct dissolution produces dense fog of Sulphuric acid which is not condensed easily hence it is first dissolved
How does liquefaction of gases work?
So, liquefaction of gases means converting those gases back to its liquid phase by simultaneously decreasing temperature and pressure and bringing them below their critical values upto a point where liquid form exists.
What is it called when gases are brought back to liquid phase?
Now to bringing back those gases back to liquid phase is called liquefaction.
Is SO3 a gas?
Yes SO3 is a gas. It is an acidic anhydride of H2SO4 (Sulphuric acid). As acidic anhydride it turns moist blue litmus red. Can be prepared :
Do real gases differ from ideal gases?
All real gases deviate from the ideal behavior but depending on the identity and the conditions, the deviation maybe significant or less significant. Real gases differ from ideal gases such that,
What is sulfur trioxide?
Sulfur trioxide, is a colorless to white crystalline solid which will fume in air. Often shipped with inhibitor to prevent polymerization. It reacts violently with water to form sulfuric acid with the release of heat. It is corrosive to metals and tissue. It causes eye and skin burns. Ingestion causes severe burns of mouth esophagus and stomach. The vapor is very toxic by inhalation. It is a fire risk when in contact with organic materials such as wood, cotton, fiberboard, etc.
How to keep sulfur trioxide from entering water?
Use dikes to prevent sulfur trioxide from entering water sources and sewers. Sulfur trioxide may be shipped domestically via air (cargo only), rail (cargo only), road, and water, in containers bearing the label, "Corrosive.". Sulfur trioxide should be stored in air-tight containers, away from moisture.
What is the reaction between sulfur trioxide and nitryl chloride?
Liquid sulfur trioxide react s violently with nitryl chloride, even at 75° C. The reaction of sulfur trioxide and lead oxide causes white luminescence [Mellor 7:654 1946-47]. The combination of iodine, pyridine, sulfur trioxide, and formamide developed a gas over pressurization after several months.
What is the reaction of sulfur trioxide and oxygen difluoride?
The reaction of SULFUR TRIOXIDE and oxygen difluoride is very vigorous and explosions occur if the reaction is carried out in the absence of a solvent [J. Chem. Eng. Data 13 (4):529-531. 1968]. The reaction of sulfur trioxide in excess with tetrafluoroethylene causes explosive decomposition to carbonyl fluoride and sulfur dioxide [Chem. Eng. News 49 (22):3. 1971]. The reaction of anhydrous perchloric acid with sulfur trioxide is violent and accompanied by the evolution of considerable heat (Pascal 16:300 1931-34). Liquid sulfur trioxide reacts violently with nitryl chloride, even at 75° C. The reaction of sulfur trioxide and lead oxide causes white luminescence [Mellor 7:654 1946-47]. The combination of iodine, pyridine, sulfur trioxide, and formamide developed a gas over pressurization after several months. This is due to the slow formation of sulfuric acid, from external water or dehydration of the formamide to hydrogen cyanide.
What is a sulfan?
Under the trademark Sulfan, a product is marketed which contains 0.5% of a stabilizer to prevent polymerization to higher melting forms: Sulfan A consists largely of beta sulfur trioxide ... Sulfan B consists largely of gamma sulfur trioxide ... Sulfan C contains no stabilizer and will polymerize to alpha sulfur trioxide.
Is sulfuric acid a corrosive substance?
/SIGNS AND SYMPTOMS/ Sulfur trioxide and sulfuric acid mists are strongly irritants, and inhaling concn of approximately 3 mg/cu m causes a choking sensation. Persons accustomed to the exposure are unable to notice concn at this order of magnitude. Sulfur trioxide is irritating and corrosive to all mucous surfaces and causes inflammation of the upper respiratory tract and possible lung injury.
Is water a strong acid?
The solution in water is a strong acid, it reacts violently with bases and is corrosive metals forming flammable/explosive gas (hydrogen). International Program on Chemical Safety/Commission of the European Communities; International Chemical Safety Card on Sulfur trioxide (October 2002).
What is SO3 used for?
When SO3 is exposed to air, it rapidly takes up water and gives off white fumes. It can react with water to form sulfuric acid. SO3 is also called sulfuric oxide and sulfuric anhydride. It is used in the production of sulfuric acid and other chemicals, and explosives. Sulfuric acid is a clear, colorless, oily liquid that is very corrosive. It is also called sulphine acid, battery acid, and hydrogen sulfate. It is used in the manufacture of fertilizers, explosives, other acids, and glue; in the purifiction of petroleum; in the pickling of metal; and in lead-acid batteries (used in most vehicles).
Is sulfuric acid corrosive?
Sulfuric acid is a clear, colorless, oily liquid that is very corrosive. It is also called sulphine acid, battery acid, and hydrogen sulfate. It is used in the manufacture of fertilizers, explosives, other acids, and glue; in the purifiction of petroleum; in the pickling of metal; and in lead-acid batteries (used in most vehicles).
What is sulfur trioxide?
Sulfur trioxide, is a colorless to white crystalline solid which will fume in air. Often shipped with inhibitor to prevent polymerization. It reacts violently with water to form sulfuric acid with the release of heat. It is corrosive to metals and tissue. It causes eye and skin burns. Ingestion causes severe burns of mouth esophagus and stomach. The vapor is very toxic by inhalation. It is a fire risk when in contact with organic materials such as wood, cotton, fiberboard, etc.
What is the reaction of sulfur trioxide and oxygen difluoride?
The reaction of SULFUR TRIOXIDE and oxygen difluoride is very vigorous and explosions occur if the reaction is carried out in the absence of a solvent [J. Chem. Eng. Data 13 (4):529-531. 1968]. The reaction of sulfur trioxide in excess with tetrafluoroethylene causes explosive decomposition to carbonyl fluoride and sulfur dioxide [Chem. Eng. News 49 (22):3. 1971]. The reaction of anhydrous perchloric acid with sulfur trioxide is violent and accompanied by the evolution of considerable heat (Pascal 16:300 1931-34). Liquid sulfur trioxide reacts violently with nitryl chloride, even at 75° C. The reaction of sulfur trioxide and lead oxide causes white luminescence [Mellor 7:654 1946-47]. The combination of iodine, pyridine, sulfur trioxide, and formamide developed a gas over pressurization after several months. This is due to the slow formation of sulfuric acid, from external water or dehydration of the formamide to hydrogen cyanide.
Why does formamide form a gas?
This is due to the slow formation of sulfuric acid, from external water or dehydration of the formamide to hydrogen cyanide.
Is sulfur trioxide corrosive?
Warning: Sulfur trioxide is extremely corrosive. Caution is advised. Signs and Symptoms of Sulfur Trioxide Exposure: Signs and symptoms of acute ingestion of sulfur trioxide may be severe and include salivation, intense thirst, difficulty in swallowing, chills, pain, and shock. Oral, esophageal, and stomach burns are common.
How to test SO3 gas?
Other method to test the existence of SO3 gas is to increase the inlet temperature of acid. In Iran, the inlet temperature of acid towers in sulfuric acid burning plants is commonly between 50-60 °C, which is not feasible to increase the temperature of inlet acid to the towers in terms of the type of pumps applied. But the optimum temperature of entering acid to the towers is estimated to be 65-85 °C.
How to test the existence of sulfur trioxide?
To test the existence of sulfur trioxide, the temperature of inlet acid to the tower can be altered and in case of the decrease of exhaust gas compared to the increase in the temperature of inlet acid to the tower, it can be identified that the issue is in the absorption of SO3 that can be solved with increasing the temperature of inlet acid to the tower or changing the pump to vertical pumps immersed in the circulation tank.
How fast is sulfur trioxide?
In sulfur trioxide absorption towers, the gas speed is 1 to 2 M/S that is chosen depending on the design, packing, gas temperature and commonly about one M/S is selected. If the rate is that the design of towers is wrong and the gas speed is higher than the standard level, this gas must be deleted from the chimney by decreasing the flow of crystals so that it is not visible.
What happens if you have steam in your gas?
If there is steam in the gas outlet, the smoke ejected will be in the form of gas and the corrosion of the chimney will increase, and if the previous gas test is negative, the major issue is water which is likely to have entered the gas from various equipment. In the following, we will investigate the present factors.
What is the best material for SO3?
Carbon steel is a suitable material of construction for handling and storing pure SO3. A corrosion allowance of 3 mm (1/8") is usually specified. If used for piping, velocites should be kept below 0.5 m/s (1.6 ft/s).
What are the three forms of sulfur trioxide?
Solid sulphur trioxide can exist in one of three forms; alpha (a), beta (b) and gamma (?) . All three forms may be present at the same time depending on conditions.
How is sulfur trioxide produced?
Sulphur trioxide is produced in a sulphuric acid plant converter from the conversion of sulphur dioxide and oxygen over a vanadium catalyst. In this case it is an intermediate product since the SO 3 produced is absorbed in concentrated sulphuric acid to produce sulphuric acid. If SO 3 itself is required, it is generally produced from oleum (i.e. fuming sulphuric acid). Oleum is heated which will drive off the free SO 3 which can then be used in the process or condensed to form pure liquid SO 3 .
What happens when sulphur trioxide is in contact with combustible materials?
A fire may result if sulphur trioxide comes in contact with combustible materials.
What happens if you melt sulphur trioxide?
If melting of solid sulphur trioxide is not done properly, over pressurization of the equipment can occur.
Does sulfur trioxide cause fog?
Even small spills of sulphur trioxide will produce a dense fog . The large size of the cloud will seem to be out of proportion to the amount of liquid spilled. Fuming is proportional to the surface area so a reduction in the surface area of the spill will reduce the amount of fuming.
Suitable products for Sulphur trioxide SO 3
Whether portable gas detectors, gas detection tubes or personal protective equipment - Dräger offers a comprehensive portfolio to protect you when handling hazardous substances.
Get in touch with Dräger
If you need further advice for the product selection or if you have not yet found what you want, get in touch with us, we'll find a solution for you.
What is sulfur trioxide?
Sulfur trioxide and oleums are strongly acidic materials that react rapidly with water to form sulfuric acid, evolving considerable heat. It can rapidly dehydrate body tissues and cause severe chemical and thermal burns.
What metals are resistant to SO3/oleum?
SO3/oleum attacks cast iron, brass, bronze and most other non-ferrous metals. Mild steel (carbon steel) and stainless steel are resistant to corrosion, and are recommended for storage systems and piping. Teflon® is the only known resistant plastic material. Rubber, neoprene, polyester, PVC, FRP and other elastomers are readily attacked and unsuitable for this service.
How to safely handle sulfur trioxide?
Use of appropriate personal protective equipment is essential to safely handle sulfur trioxide and oleum. Do not get sulfur trioxide or oleums in eyes, on skin or on clothing. Do not breathe vapors or mists and use with adequate ventilation. Wash hands thoroughly after handling. Remove contaminated clothing or shoes immediately. Wash clothing before reuse.
What is required for SO3/oleum spills?
All sites handling SO3/oleum must have equipment and trained personnel available to render spills/leaks non-fuming, neutralize the spill , and provide for the proper disposal of the neutralized acids. Personnel must be familiar in firefighting and handling procedures in order to proceed with cleanup.
Can water be added to SO3?
Water or caustic solutions should never be directly added to SO3/oleums in an uncontrolled fashion, or by untrained personnel, because of the potential for a violent reaction and subsequent spattering. When diluting, SO3/oleum may be added to strong sulfuric acid while mixing and cooling to remove the heat of dilution.
Is sulfur trioxide a hygroscopic substance?
Sulfur trioxide and oleum have a strong, irritating, acrid odor and are very hygroscopic. They react violently with water, so uncontrolled contact with any aqueous system should be avoided. They are oxidizing agents and may cause ignition by contact with combustible materials.
Is sulfuric acid an inorganic or inorganic acid?
Sulfuric acid is one of the oldest known industrial chemicals. It is a very strong inorganic acid with qualities that make it very useful for a number of industries. More sulfuric acid is produced and consumed than any other chemical in the world. Sulfur trioxide and oleum are the strongest inorganic acid produced from sulfur. Some of the industries that find sulfur trioxide and oleum essential include: