Is SO2 (sulfur dioxide) soluble in water?
Answer: SO2 ( Sulfur dioxide ) is Soluble in water. What is Soluble and Insoluble ? Solubility. Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent.
What is the solubility of sulfur in water?
Sulfur would be very sparingly (or for simplicity's sake, insoluble) in water. To understand this, we would need to see what type of interaction sulfur and water undergo respectively. To do this, we need to explore the different structures of sulfur and water. Sulfur exists as S8, a simple molecule, in its elemental form.
What happens when sulphur dioxide reacts with water?
sulphur dioxide is very soluble in water. when water is added to sulphur dioxide ( i.e. sulphur dioxide react with water or rather SO2 is bubbled through water ) , H2SO3 is formed. that is a reason why SO2 is blamed as the key gas for causing acid rain.
What is sulfur dioxide?
Sulfur dioxide is the product of the burning of sulfur or of burning materials that contain sulfur: To aid combustion, liquified sulfur (140–150 °C, 284-302 °F) is sprayed through an atomizing nozzle to generate fine drops of sulfur with a large surface area.
See more

Is sulphur dioxide highly soluble in water?
Sulfur dioxide has very high solubility in water and is considered to be a highly corrosive compound under atmospheric conditions.
How is sulfur dioxide soluble in water?
It is readily soluble in cold water, sparingly soluble in hot water, and soluble in alcohol, acetic acid, and sulfuric acid. It is corrosive to organic materials and dissolves in water to form sulfurous acid, H2SO3.
Is sulphur soluble in water?
Elemental sulfur is known to be insoluble in water. However, in certain processes, such as geological processes, where time compensates for the very small concentration, the solubility of sulfur in water can be of interest.
What happens when sulphur dioxide is dissolved in water?
Complete answer: When sulfur dioxide is dissolved in water it forms sulphurous acid. When sulphur dioxide is dissolved in water it forms sulfurous acid.
Is SO2 polar or nonpolar?
CO2 is linear so its dipoles cancel. However, since SO2 is bent, the dipoles do not cancel and the molecule is polar.
Is sulfur dioxide more soluble in hot or cold water?
Sulfur dioxide is a colorless gas (a liquid below −10 °C or under pressure) with a characteristic, pungent odor, more soluble in cold than in hot water.
Why sulfur molecules are not soluble in water?
The same principles govern the solubilities of molecular solids in liquids. For example, elemental sulfur is a solid consisting of cyclic S 8 molecules that have no dipole moment. Because the S 8 rings in solid sulfur are held to other rings by London dispersion forces, elemental sulfur is insoluble in water.
Can sulphur mix with water?
Sulfur enters into a reaction with oxygen and with complex substances (halogens, acids) as a reducer. Sulfur does not dissolve in water in ordinary conditions.
How do you make sulphur water soluble?
0:274:35Dissolving sulfur in water - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd make sure the potassium hydroxide solution is boiling hot then in this sped up footage andMoreAnd make sure the potassium hydroxide solution is boiling hot then in this sped up footage and adding sulfur in three portions. When no more sulfur dissolves.
Which acid is produced when SO2 is dissolved in water?
Therefore, sulphurous acid (H2SO3) is formed when sulphur dioxide dissolves in water.
What type of reaction is sulfur dioxide and water?
Type of Chemical Reaction: For this reaction we have a combination (also called synthesis) reaction.
When sulphur dioxide is dissolved in water ___ acid is formed?
The acid formed when sulphur dioxide dissolves in water is sulphurous acid H2SO3.
What's the solubility of sulfur dioxide?
WaterSulfur dioxide / Soluble inWater is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms. It is vital for all known forms of life, despite providing neither food, energy, nor organic micronutrients. Wikipedia
Does SO2 react with water?
Sulfur dioxide dissolves in water to give solutions that contain sulfurous acid, H2SO3. The following equilibria are established by sulfurous acid, which is a weak diprotic acid known only in solution.
What is solubility of CO2 in water?
WaterCarbon dioxide / Soluble inWater is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms. It is vital for all known forms of life, despite providing neither food, energy, nor organic micronutrients. Wikipedia
What forms when CO2 dissolves in water?
When carbon dioxide reacts with water, carbonic acid is formed, from which hydrogen ions dissociate, increasing the acidity of the system.
Where is sulfur dioxide found?
Sulfur dioxide is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm. On other planets, sulfur dioxide can be found in various concentrations, the most significant being the atmosphere of Venus, where it is the third-most abundant atmospheric gas at 150 ppm.
How is sulfur dioxide made?
Most sulfur dioxide is produced by the combustion of elemental sulfur. Some sulfur dioxide is also produced by roasting pyrite and other sulfide ores in air.
What is the primary source of sulfur dioxide?
On both Venus and Mars, as on Earth, its primary source is thought to be volcanic. The atmosphere of Io, a natural satellite of Jupiter, is 90% sulfur dioxide and trace amounts are thought to also exist in the atmosphere of Jupiter .
How much sulfur dioxide was in the atmosphere in 1999?
1999. 18,867,000 short tons (17.1 Mt) Sulfur dioxide is a major air pollutant and has significant impacts upon human health. In addition, the concentration of sulfur dioxide in the atmosphere can influence the habitat suitability for plant communities, as well as animal life.
What is the effect of sulfuric acid on copper turnings?
On a laboratory scale, the action of hot concentrated sulfuric acid on copper turnings produces sulfur dioxide.
What bonding mode is sulfur dioxide?
As a η 1 -SO 2 (S-bonded planar) ligand sulfur dioxide functions as a Lewis base using the lone pair on S. SO 2 functions as a Lewis acids in its η 1 -SO 2 (S-bonded pyramidal) bo nding mode with metals and in its 1:1 adducts with Lewis bases such as dimethylacetamide and trimethyl amine. When bonding to Lewis bases the acid parameters of SO 2 are E A = 0.51 and E A = 1.56.
What is the bond order of sulfur and oxygen?
The sulfur–oxygen bond has a bond order of 1.5. There is support for this simple approach that does not invoke d orbital participation. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1.
Why is sulfur not soluble in water?
Because it normally it exist as S8. which is a stable yellow solid. In order for molecule to solvate in water it must break some of its bonds and make new bonds with H20. Since S8 is stable, attractions with water molecule are not enough to break the bonds of S8. So sulfur isn’t soluble in water.
Why does sulphur not react with water?
Why? Sulphur is hydrophobic meaning it does not like water in fact it is fairly difficult to find a solvent that readily does dissolve sulphur except for some organic solvents like toluene or perchlorethylene or carbon disulphide, with CS2 being the best. Sulphur also under standard conditions forms an eight atom ring and has an oxidation potential of 0. Because of this neutral potential it is not attracted to the polar solvent water and this is the reason for its lack of desire to play nice with water but chlorine does.
What is the reaction between sulfur and water?
For compounds to mix together (in this case solute and solvent), the reaction must be energetically favourable. For the reaction to be energetically favourable, the solution must be exothermic - which lowers the energy of the system and thus creates a more stable solution than the solute/ solvent. However, since the (theoretical) solution between sulfur and water would lead to at best a pd-pd interaction (which is less stable and less strong than h-bond), it is energetically unfeasible.
How many electrons does chlorine have?
It is all about valence electrons and polarity. These electrons are the ones in the outer most orbital shell of the atom and there can be as many as eight electrons in this shell. A single Chlorine atom reacts very well because it has a single electron in its outer shell, it is polar and it would like to donate it quite badly so it reacts with something to do it. It so happens that sulphur only needs two electrons to fill its outer shell and likes to react as well, and does with many elements and compounds, but water is not one. Normally chlorine is in a pair Cl2 and not that reactive with wat
Why is H2O connected to water?
However, water, H2O exists as simple molecules but are all connected to one another via hydrogen bonding. This is because oxygen is electronegativ. Continue Reading.
Why is water not miscible in oil?
Water is not miscible in oil or other nonpolar liquids not because they repel but because the attractive cohesive forces between water molecules are stronger than the weak dispersion (adhesive) forces between water and the nonpolar liquid.
What is the reaction of a compound to a solute and solvent?
For compounds to mix together (in this case solute and solvent), the reaction must be energetically favourable. For the reaction to be energetically favourable, the solution must be exothermic - which lowers the energy of the system and thus creates a more stable solution than the solute/ solvent.

Overview
Uses
The overarching, dominant use of sulfur dioxide is in the production of sulfuric acid.
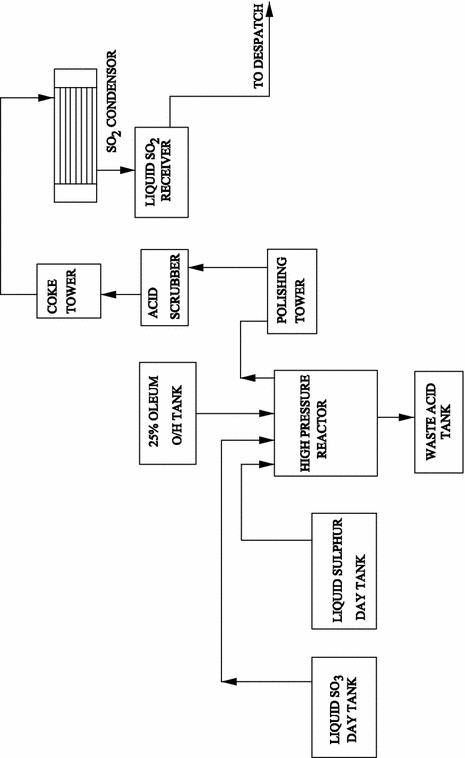
Sulfur dioxide is an intermediate in the production of sulfuric acid, being converted to sulfur trioxide, and then to oleum, which is made into sulfuric acid. Sulfur dioxide for this purpose is made when sulfur combines with oxygen. Th…
Structure and bonding
SO2 is a bent molecule with C2v symmetry point group. A valence bond theory approach considering just s and p orbitals would describe the bonding in terms of resonance between two resonance structures.
The sulfur–oxygen bond has a bond order of 1.5. There is support for this simple approach that does not invoke d orbital participation. In terms of electron-coun…
Occurrence
Sulfur dioxide is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm.
On other planets, sulfur dioxide can be found in various concentrations, the most significant being the atmosphere of Venus, where it is the third-most abundant atmospheric gas at 150 ppm. There, it reacts with water to form clo…
Production
Sulfur dioxide is primarily produced for sulfuric acid manufacture (see contact process). In the United States in 1979, 23.6 million metric tons (26 million U.S. short tons) of sulfur dioxide were used in this way, compared with 150,000 metric tons (165,347 U.S. short tons) used for other purposes. Most sulfur dioxide is produced by the combustion of elemental sulfur. Some sulfur dioxide is also produced by roasting pyrite and other sulfide ores in air.
Reactions
Featuring sulfur in the +4 oxidation state, sulfur dioxide is a reducing agent. It is oxidized by halogens to give the sulfuryl halides, such as sulfuryl chloride:
SO2 + Cl2 → SO2Cl2
Sulfur dioxide is the oxidising agent in the Claus process, which is conducted on a large scale in oil refineries. Here, sulfur dioxide is reduced by hydrogen sulfid…
As an air pollutant
Sulfur dioxide is a noticeable component in the atmosphere, especially following volcanic eruptions. According to the United States Environmental Protection Agency (EPA), the amount of sulfur dioxide released in the U.S. per year was:
Sulfur dioxide is a major air pollutant and has significant impacts upon human health. In addition, the concentration of sulfur dioxide in the atmosphere can influence the habitat suitability for pla…
Safety
Incidental exposure to sulfur dioxide is routine, e.g. the smoke from matches, coal, and sulfur-containing fuels.
Sulfur dioxide is mildly toxic and can be hazardous in high concentrations. Long-term exposure to low concentrations is also problematic. A 2011 systematic review concluded that exposure to sulfur dioxide is associated with preterm birth