What are the element that are classified as metalloids?
The six elements that are unanimously considered to be metalloids are the following: Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium. Apart from these six elements, the definition of metalloid elements sometimes includes the elements bismuth, polonium, and astatine as well.
Is a metalloid the same as a semimetal?
The term metalloid originally referred to nonmetals. Its more recent meaning, as a category of elements with intermediate or hybrid properties, became widespread in 1940–1960. Metalloids are sometimes called semimetals, a practice that has been discouraged, as the term semimetal has a different meaning in physics than in chemistry
Is a metalloid a metal or a non metal?
Metalloids. Meaning. The metals which exhibit the highest degree of metallic behaviour is known as metals. Non-metals are such elements which do not possess any metallic behaviour. Metalloids are such elements, which possess some of the properties like metal, while some like non-metal.
Can you give examples of elements that are metalloids?
Metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. An element that isn’t a metal yet has certain metal-like qualities. Boron, silicon, germanium, arsenic, antimony, tellurium, and polonium are examples of metalloids.
What element is a metalloid?
The term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic, antimony, tellurium, and possibly bismuth, polonium, astatine) found near the center of the P-block or main block of the periodic table.
Which group of elements contains a metalloid?
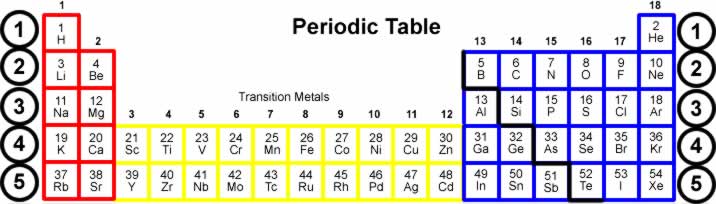
Groups 13–16 of the periodic table (orange in the Figure below) are the only groups that contain elements classified as metalloids. Unlike other groups of the periodic table, which contain elements in just one class, groups 13–16 contain elements in at least two different classes.
How do you know if an element is a metalloid?
The metals are to the left of the line (except for hydrogen, which is a nonmetal), the nonmetals are to the right of the line, and the elements immediately adjacent to the line are the metalloids.
What are the 8 elements considered metalloids?
Frequently Asked Questions on Metalloids They are: antimony (Sb), germanium (Ge), silicon (Si), arsenic (As), tellurium (Te), polonium (Po), boron (B), and astatine (At).
What are metalloids examples?
Metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium. An element that isn't a metal yet has certain metal-like qualities. Boron, silicon, germanium, arsenic, antimony, tellurium, and polonium are examples of metalloids.
Which is not a metalloid?
Beryllium is not a metalloid. While others, i.e., silicon, germanium, and arsenic are metalloids.
How do you know if an element is a metal nonmetal or metalloid?
Elements to the left of the line are considered metals. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. Elements to the far right of the periodic table are nonmetals.
Where are the metalloids?
Metalloids belong to p-block elements and it is placed on the right side of the periodic table. Metalloids exhibit the properties of both metals as well as non-metals.
How many metalloids are on the periodic table?
6The number of metalloids in the modern periodic table are 6. The names are : boron, silicon, germanium, polonium, tellurium, arsenic.
Which element is a metalloid quizlet?
A metalloid is an element that has physical and chemical properties of both metals and nonmetals. The elements boron, silicon, germanium, arsenic, antimony, tellurium, polonium, and astatine are metalloids.
What are 5 properties of metalloids?
Five Main Properties of MetalloidsProperties intermediate between metals and nonmetals.Physical appearance similar to metals.Semi-conductors of electricity.Brittle.Chemical properties are more similar to nonmetals than to metals.
Is carbon a metalloid?
Carbon has 4 electrons in its valence shell which makes it a metalloid but commonly it is considered as a non-metal.
Where are metalloids found?
Metalloids are located between the metals and nonmetals. The orange color on the Periodic table represents metalloids. They form a separating boundary between the metals and nonmetals.
Which list of elements contains two metalloids?
Group 14 is called the carbon group. This group contains two metalloids: silicon and germanium.
What element is in group 13 Period 2?
The boron group are the chemical elements in group 13 of the periodic table, comprising boron (B), aluminium (Al), gallium (Ga), indium (In), thallium (Tl), and perhaps also the chemically uncharacterized nihonium (Nh)....Boron group.Mercury (element)ThalliumLeadBismuthPolonium6 more columns
What are the elements that are classified as metalloids?
Only the elements at or near the margins, lacking a sufficiently clear preponderance of either metallic or nonmetallic properties, are classified as metalloids.
What is a metalloid?
v. t. e. A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids.
What are the two names for metalloids?
The word metalloid comes from the Latin metallum ("metal") and the Greek oeides ("resembling in form or appearance"). Several names are sometimes used synonymously although some of these have other meanings that are not necessarily interchangeable: amphoteric element, boundary element, half-metal, half-way element, near metal, meta-metal, semiconductor, semimetal and submetal. "Amphoteric element" is sometimes used more broadly to include transition metals capable of forming oxyanions, such as chromium and manganese. " Half-metal " is used in physics to refer to a compound (such as chromium dioxide) or alloy that can act as a conductor and an insulator. "Meta-metal" is sometimes used instead to refer to certain metals ( Be, Zn, Cd, Hg, In, Tl, β-Sn, Pb) located just to the left of the metalloids on standard periodic tables. These metals are mostly diamagnetic and tend to have distorted crystalline structures, electrical conductivity values at the lower end of those of metals, and amphoteric (weakly basic) oxides. "Semimetal" sometimes refers, loosely or explicitly, to metals with incomplete metallic character in crystalline structure, electrical conductivity or electronic structure. Examples include gallium, ytterbium, bismuth and neptunium. The names amphoteric element and semiconductor are problematic as some elements referred to as metalloids do not show marked amphoteric behaviour (bismuth, for example) or semiconductivity (polonium) in their most stable forms.
How many metalloids are there?
The number and identities of metalloids depend on what classification criteria are used. Emsley recognised four metalloids (germanium, arsenic, antimony, and tellurium); James et al. listed twelve (Emsley's plus boron, carbon, silicon, selenium, bismuth, polonium, moscovium, and livermorium ). On average, seven elements are included in such lists; individual classification arrangements tend to share common ground and vary in the ill-defined margins.
Which elements are toxic?
All six of the elements commonly recognised as metalloids have toxic, dietary or medicinal properties. Arsenic and antimony compounds are especially toxic; boron, silicon, and possibly arsenic, are essential trace elements. Boron, silicon, arsenic, and antimony have medical applications, and germanium and tellurium are thought to have potential.
What is a period of metalloids?
Periods (1–7, ...) Blocks (s, p, d, f, ...) A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids.
Where are metaloids located?
Metalloids lie on either side of the dividing line between metals and nonmetals. This can be found, in varying configurations, on some periodic tables. Elements to the lower left of the line generally display increasing metallic behaviour; elements to the upper right display increasing nonmetallic behaviour. When presented as a regular stairstep, elements with the highest critical temperature for their groups (Li, Be, Al, Ge, Sb, Po) lie just below the line.
How to determine if an element is metalloid?
The easiest way to decide if a given unknown element is a metalloids is by testing whether any metal and non-metal characteristics can be detected . If both are detected, then the given element is most likely to be a metalloid element.
What are Metalloids?
Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and the non metal categories. Boron, germanium, silicon, antimony, arsenic, and tellurium are the six most widely recognized metalloids. Apart from these elements, the following elements are also known to be classified as metalloids in some circumstances:
What are the elements that are found in the step-like line between metals and nonmetals?
They are: antimony (Sb), germanium (Ge), silicon (Si), arsenic (As), tellurium (Te), polonium (Po), boron (B), and astatine (At).
What element is used to form alloys?
This element also has the ability to form alloys with these MnB composition metals if the value of n is greater than 2. In fact, ferroboron (which contains 15 per cent boron) is widely used in order to inject boron into steel. Furthermore, nickel-boron alloys are used as ingredients in the engineering industry for welding alloys and case hardening compositions.
What is the dividing line between metals and nonmetals?
Some periodic tables have a dividing line between metals and nonmetals, and below this line, the metalloids can be found. Typically, metalloids have metallic appearances but they are usually brittle and only mediocre electricity conductors. Chemically, these elements usually behave as non-metals. Metalloids have the ability to form metallic alloys.
What are the elements that are used in medicine?
However, boron, arsenic, and silicon are extremely important trace elements.The four elements boron, arsenic, silicon, and antimony are known to have many medical uses. The remaining two elements (germanium and tellurium) are known to have great potential for medicinal applications. Furthermore, boron is used in herbicides ...
What is the most common alloy used in automotive?
Silicon alloys of aluminium and iron are widely used in the construction and automotive industries. Germanium is known to form several alloys, especially with the coinage metals in particular.
Which type of metalloid has the physical properties of metals?
In general, metalloids have the physical properties of metals, but their chemical properties are closer to those of nonmetals: Semimetals tend to make excellent semiconductors, although most of the elements themselves are not technically semiconducting.
What is a metalloid?
Semimetals or metalloids are chemical elements that have properties of both metals and nonmetals. Metalloids are important semiconductors, often used in computers and other electronic devices.
What is a semimetal compound?
Some texts use the terms semimetals and metalloids interchangeably, but more recently, the preferred term for the element group is "metalloids," so that "semimetals" may be applied to chemical compounds as well as elements that exhibit properties of both metals and nonmetals. An example of a semimetal compound is mercury telluride (HgTe).
What are the physical properties of metalloids?
In general, metalloids have the physical properties of metals, but their chemical properties are closer to those of nonmetals: 1 Semimetals tend to make excellent semiconductors, although most of the elements themselves are not technically semiconducting. Exceptions are silicon and germanium, which are true semiconductors, as they can conduct electricity under the right conditions. 2 These elements have lower electrical and thermal conductivity than metals. 3 Semimetals/metalloids have high lattice dielectric constants and high diamagnetic susceptibilities. 4 Semimetals are typically malleable and ductile. One exception is silicon, which is brittle. 5 Metalloids may either gain or lose electrons during chemical reactions. Oxidation numbers of elements in this group range from +3 to -2. 6 As far as appearances go, metalloids range from dull to shiny. 7 Metalloids are extremely important in electronics as semiconductors, although they are also used in optical fibers, alloys, glass, and enamels. Some are found in drugs, cleaners, and pesticides. The heavier elements tend to be toxic. Polonium, for example, is dangerous due to its toxicity and radioactivity.
Where are semimetals found?
Semimetal or Metalloid Properties. Semimetals or metalloids are found in a zig-zag line on the periodic table, separating the basic metals from the nonmetals. However, the defining characteristic of metalloids is not so much their position on the periodic table as the extremely small overlap between the bottom of the conduction band and top ...
Where are metalloids found?
On the periodic table, metalloids are found along a zig-zag line between boron and aluminum down to polonium and astatine.
What are metaloids used for?
Metalloids are used to make semiconductors, ceramics, polymers, and batteries. Metalloids tend to be shiny, brittle solids that act as insulators at room temperature but as conductors when heated or combined with other elements.
What are Metalloid Elements?
Metalloid elements, also known as semimetals, are elements that have properties of both metals and nonmetals. The metalloid definition is considered to include between six to nine elements that occur along a slanted line between the metal and nonmetal elements of the periodic table. The six elements that are unanimously considered to be metalloids are the following:
What are the elements that are considered metalloids?
The six elements that are unanimously considered to be metalloids are the following: Boron. Silicon. Germanium. Arsenic. Antimony. Tellurium. Apart from these six elements, the definition of metalloid elements sometimes includes the elements bismuth, polonium, and astatine as well.
How many types of metalloids are there?
There are three distinct categories of metalloid elements based on the number of valence electrons, and the chemical properties within each category are fairly similar.
Why are metalloids ambiguous?
This ambiguity is in large part due to a lack of specific properties that are considered characteristics of all metalloids. Instead, the metalloid elements are simply characterized as having a mix of properties that are in between the properties of metals and nonmetals.
Where are metalloids located?
The metalloids are located along a slanted line between the metal elements and nonmetal elements of the periodic table. They span from Group 13 to Group 16, 17, or 18 based on what criteria of classifying metalloid elements is being used.
Where are metalloids on the periodic table?
As previously mentioned, metalloids are a group of elements that occur in a slanted line between the metals and nonmetals on the periodic table. This line of metalloid elements spans between Group 13 to Group 16, 17, or 18 (depending on how many elements are considered to be metalloids truly).
Which element has four valence electrons?
There are also two metalloid elements - silicon and germanium - which have exactly four valence electrons. These elements can act either as a metal (by giving up electrons) or nonmetal (by accepting electrons) depending on the other elements involved in the chemical reaction.
What are the elements that are considered metalloids?
One or more of carbon, aluminium, phosphorus, selenium, tin or bismuth, these being periodic table neighbours of the elements commonly classified as metalloids, are sometimes recognised as metalloids.
How many elements are in a metalloid?
There is an average of 7.15 elements per metalloid list.
What does the parenthesized symbol mean in a metalloid list?
A parenthesized symbol indicates an element whose inclusion in a particular metalloid list is qualified in some way by the author (s). The 'citations' rows show how many and what percentage of the authorities consider each element to be a metalloid, with qualified citations counted as one-half.
What are metalloids?
Elements regarded as metalloids. The elements commonly classified as metalloids are boron, silicon, germanium, arsenic, antimony and tellurium. The status of polonium and astatine is not settled.
Is Selenium a metalloid?
Selenium, in particular, is commonly designated as a metalloid in environmental chemistry on account of similarities in its aquatic chemistry with that of arsenic and antimony. There are fewer references to beryllium, in spite of its periodic table position adjoining the dividing line between metals and nonmetals.
How many elements are metalloid?
As mentioned above, there are 7 elements in the periodic table that exhibit metalloid behavior. They occur in a diagonal line from boron to astatine through the p-block. The elements in the upper-right portion of the line show increasing non-metallic behavior and the elements at the lower-left of the line show increasing metallic behavior.
What are metalloids?
Metalloids are elements from the periodic table with properties that lie between metals and non-metals. The following ScienceStruck article will cover some information related to metalloids. The first person to come up with a periodic table of elements was Dmitri Ivanovich Mendeleev, a Russian chemist. He came up with the first version of periodic ...
What is the line that separates metalloids from non-metals called?
The line that separates metalloids from the metals and non-metals in the periodic table is called amphoteric line.
What is the meaning of the term "metalloid"?
The term metalloid comes from the Greek word metallon, which means ‘metal’, and edios, meaning ‘sort.’ The metalloids are often seen forming amphoteric oxides, and they behave as semiconductors. They have properties of both metals and non-metals in the periodic table. They even carry electric charge that makes them suitable for use in computers and calculators. Their ionization energy as well as electronegativity values are between those of metals and non-metals. Their reactivity depends on the metals that they are reacting with.
How do the chemical properties of elements vary?
According to Mendeleev’s law of periodic table, the chemical and physical properties of elements vary in a periodic fashion according to their atomic weights. However, the modern periodic table of elements follow the law that, the properties of elements vary according to their atomic number and not by their weight. The elements of a Mendeleev’s table were arranged in rows called periods and columns called groups. The chemical elements of the same group had similar properties. There are different regions in the periodic table that are called periodic table blocks, as they are named according to the subshell of the last electron of the atom.
Where are metalloids located?
Location of Metalloids in the Periodic Table. The metalloids, also known as semi-metals, are placed between metals and non-metals in the periodic table of elements. There are seven elements that are classified as metalloids and placed in Group 13, 14, 15, 16, and 17. They are found in a stair step line that helps differentiate metals ...
Where does the term "metalloid" come from?
The term metalloid comes from the Greek word metallon, which means ‘metal’, and edios, meaning ‘sort.’. The metalloids are often seen forming amphoteric oxides, and they behave as semiconductors. They have properties of both metals and non-metals in the periodic table.
Tellurium
Tellurium (Te) Introduction Tellurium is a chemical element with the atomic number 52 in the periodic table. It’s an extremely rarely found element in Earth’s crust. Its occurrence can be compared with that of the element platinum.
Arsenic
Arsenic (Ar) Introduction Arsenic is a chemical element with an atomic number 33 in the periodic table of elements. There’s 1.5–2 ppm of this crystalline metalloid in the Earth’s crust.
Silicon
Silicon (Si) Introduction Silicon is a chemical element with the atomic number 14 in the periodic table. With an abundance of 27.7% in Earth’s crust, it’s the second most abundant chemical element.
Boron
Boron (B) Introduction Boron is a metalloid with an atomic number of 5 in the periodic table of elements. It’s found in an amount of 0.00086 percent in the Earth’s crust. Being a member of the boron family of periodic table elements, this chemical substance has three valence electrons. While boron is labeled as … Read more
Polonium
Polonium (Po) Introduction Polonium is a chemical element with the atomic number 84 in the periodic table. It’s a rare and highly radioactive metal. The natural abundance of element 84 in Earth’s crust is only 2 × 10 −10 milligrams per kilogram. Being a member of the chalcogen family of the periodic table, this … Read more
Antimony
Antimony (Sb) Introduction Antimony is a chemical element with atomic number 51 in the periodic table. With an estimated 0.00002% to 0.0005% ppm natural abundance, antimony is the 62nd most plentiful substance found in Earth’s crust.
Germanium
Introduction Germanium is a chemical element with an atomic number of 32 in the periodic table. There’s about 1.6 ppm of this substance in Earth’s crust. Being a member of the carbon family of elements, this metalloid has four valence electrons, low toxicity, and is transparent to infrared radiation.
Overview
Definitions
A metalloid is an element that possesses a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals, and which is therefore hard to classify as either a metal or a nonmetal. This is a generic definition that draws on metalloid attributes consistently cited in the literature. Difficulty of categorisation is a key attribute. Most elements have a mixture of metallic and nonmetallic properties, and can be classified according to which set of propertie…
Periodic table territory
Metalloids lie on either side of the dividing line between metals and nonmetals. This can be found, in varying configurations, on some periodic tables. Elements to the lower left of the line generally display increasing metallic behaviour; elements to the upper right display increasing nonmetallic behaviour. When presented as a regular stairstep, elements with the highest critical temperature for their groups (Li, Be, Al, Ge, Sb, Po) lie just below the line.
Properties
Metalloids usually look like metals but behave largely like nonmetals. Physically, they are shiny, brittle solids with intermediate to relatively good electrical conductivity and the electronic band structure of a semimetal or semiconductor. Chemically, they mostly behave as (weak) nonmetals, have intermediate ionization energies and electronegativity values, and amphoteric or weakly acidic oxides. They can form alloys with metals. Most of their other physical and chemical prop…
Common applications
The focus of this section is on the recognised metalloids. Elements less often recognised as metalloids are ordinarily classified as either metals or nonmetals; some of these are included here for comparative purposes.
Metalloids are too brittle to have any structural uses in their pure forms. They and their compounds are used as (or in) alloying components, biological agent…
Nomenclature and history
The word metalloid comes from the Latin metallum ("metal") and the Greek oeides ("resembling in form or appearance"). Several names are sometimes used synonymously although some of these have other meanings that are not necessarily interchangeable: amphoteric element, boundary element, half-metal, half-way element, near metal, meta-metal, semiconductor, semimetal and submetal. "Amphoteric element" is sometimes used more broadly to include transition metals ca…
Elements commonly recognised as metalloids
Properties noted in this section refer to the elements in their most thermodynamically stable forms under ambient conditions.
Pure boron is a shiny, silver-grey crystalline solid. It is less dense than aluminium (2.34 vs. 2.70 g/cm ), and is hard and brittle. It is barely reactive under normal conditions, except for attack by fluorine, and has a melting point …
Elements less commonly recognised as metalloids
Carbon is ordinarily classified as a nonmetal but has some metallic properties and is occasionally classified as a metalloid. Hexagonal graphitic carbon (graphite) is the most thermodynamically stable allotrope of carbon under ambient conditions. It has a lustrous appearance and is a fairly good electrical conductor. Graphite has a layered structure. Each layer consists of carbon ato…
What Are metalloids?
- Metalloids can be defined as chemical elements whose physical and chemical properties fall in between the metal and the non metal categories. Boron, germanium, silicon, antimony, arsenic, tellurium and pollanium are the seven most widely recognized metalloids. It can be noted that all seven of these elements can be found on the regular periodic tab...
General Properties of Metalloids
- Metalloids typically look like metals. However, these elements often behave like non-metals.
- Physically, metalloids are brittle, somewhat shiny substances that are usually solid at ambient temperatures.
- These elements usually have intermediate to fairly strong electrical conductivity
- Metalloids are known to have electronic band structures that are similar to semimetals or se…
- Metalloids typically look like metals. However, these elements often behave like non-metals.
- Physically, metalloids are brittle, somewhat shiny substances that are usually solid at ambient temperatures.
- These elements usually have intermediate to fairly strong electrical conductivity
- Metalloids are known to have electronic band structures that are similar to semimetals or semiconductors.
Applications of Metalloids
- Metalloids and the compounds of metalloids are widely used as alloys (or in the production of alloys as a component of the mixture), biological agents (which can be nutritional, toxicological, and medicinal as well), flame retardants, catalysts, glasses (which can be oxides or metallic in nature), and optical storage media. Metalloids are also known to have applications in optoelectr…