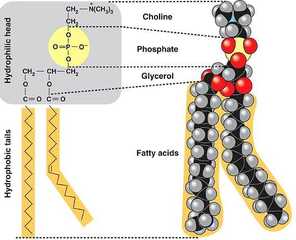
What is hydrophilicity of molecules?
Hydrophilic molecules or Hydrophilic moieties are basically polar compounds that have ionic groups. The polar nature of these hydrophilic molecules enables them to readily absorb water or polar solvent and eventually getting dissolved in polar solvents like water. What causes hydrophilicity?
Which functional group is more hydrophilic?
Which functional group is more hydrophilic? Following three functional groups are on a polymeric surface: 1. Carboxylic acid: - (C=O)-OH 2. Amide: - (C=O)-N- 3. Anhydride: - (C=O)-O- (C=O)- I though that more carboxylic acid group on the surface lead to more hydrophilic surface.
Can a molecule be hydrophobic if it has polar bonds?
Even if a molecule has polar covalent bonds, if these bonds are arranged symmetrically, the molecule overall will be hydrophobic. What makes a nonpolar molecule hydrophobic?
Does more carboxylic acid on the surface lead to more hydrophilic surface?
I though that more carboxylic acid group on the surface lead to more hydrophilic surface. However, water contact angle test shows that the surface with more anhydride group has a smaller contact angle (more hydrophilic surface). Is the contact angle result reasonable? Join ResearchGate to ask questions, get input, and advance your work.

Which functional groups are hydrophobic?
Functional groups can be classified as hydrophobic or hydrophilic based on their charge and polarity characteristics. The only hydrophobic group below is the methyl (CH 3start subscript, 3, end subscript) group, which is nonpolar.
Which functional groups are hydrophobic and hydrophilic?
Classifying Functional Groups An example of a hydrophobic group is the non-polar methane molecule. Among the hydrophilic functional groups is the carboxyl group found in amino acids, some amino acid side chains, and the fatty acid heads that form triglycerides and phospholipids.
Is a hydroxyl group always polar?
Hydroxyl R-OH The oxygen atom is much more electronegative than either the hydrogen or the carbon, which will cause the electrons in the covalent bonds to spend more time around the oxygen than around the C or H. Therefore, the O-H and O-C bonds in the hydroxyl group will be polar covalent bonds.
Is a hydroxyl group water soluble?
Hydroxyl groups are also able to form hydrogen bonds with water, a property that increases the hydrophilicity and solubility of molecules containing them. The carbohydrates are an example of a group of molecules that are extremely soluble due to hydroxyl functional groups.
Are all functional groups hydrophilic?
Functional groups are usually classified as hydrophobic or hydrophilic depending on their charge or polarity. An example of a hydrophobic group is the non-polar methane molecule.
What group makes a molecule hydrophilic?
Hydroxyl groups (-OH), found in alcohols, are polar and therefore hydrophilic (water liking) but their carbon chain portion is non-polar which make them hydrophobic. The molecule increasingly becomes overall more nonpolar and therefore less soluble in the polar water as the carbon chain becomes longer.
What are the characteristics of a hydroxyl group?
Hydroxyl groups are a functional group found in sugars and alcohols. A hydroxyl group consists of one hydrogen and one oxygen atom and can be written as either -OH or HO-. Hydroxyl groups are polar, and the oxygen side is always negative, while the hydrogen side is always positive.
What is in a hydroxyl group?
Hydroxyl groups are simple structures consisting of an oxygen atom with two lone pairs bonded to a hydrogen atom. They readily participate in hydrogen bonding, generating either a net positively or negatively charged ion.
Does water have a hydroxyl group?
alcohols. …properties because water molecules contain hydroxyl groups that can form hydrogen bonds with other water molecules and with alcohol molecules, and likewise alcohol molecules can form hydrogen bonds with other alcohol molecules as well as with water.
Why hydroxyl is soluble in water?
Due to the high electronegativity of the oxygen atom, the bond between the oxygen and the hydrogen is highly polar. Consequently, the hydroxyl group strongly attracts water molecules, forming hydrogen bonds. Hydroxyl groups are responsible for the solubility of some molecules such as sugars.
Which functional groups are water soluble?
1) Water soluble: Typically low molecular weight compounds which contain a polar functional group are water soluble. Alcohols, aldehydes, amides, amines, carboxylic acid, esters, ethers, ketone and nitriles containing up 1-5 carbons are water soluble.
How do hydroxyl groups interact with water?
Hydroxyls can form hydrogen bonds with water molecules, which cause stronger interaction between water molecules and the solid surface and accordingly lower the contact angle.
Which functional group is the most hydrophilic?
The best hydrophilic group is found in OH- group.
Are amines hydrophilic or hydrophobic?
Several tertiary amines serve as switchable-hydrophilicity solvents , meaning that they are hydrophobic solvents having very low miscibility with water when under air but hydrophilic solvents having complete miscibility with water when under an atmosphere of CO2 .
Which of the following functional groups is hydrophobic quizlet?
Methyl group. It is the only hydrophobic group.
Is ketone hydrophilic or hydrophobic?
Consequently, esters and ketones bearing typical polar groups are not classified into hydrophilic compounds, but into “hydroneutral” compounds positioned between hydrophilic and hydrophobic ones.
What causes hydrophilicity?
A hydrophilic molecule or substance is attracted to water. … This is caused by the attraction of water molecules to the hydrophilic molecules. In areas of high concentration of the molecules, water moves in and pulls the molecules apart.
Are all nonpolar molecules hydrophobic?
Nonpolar molecules are hydrophobic; “hydro-” means water and “-phobic” means fear. Nonpolar molecule are water fearing and do not easily dissolve in water. These molecules have nonpolar covalent bonds or polar covalent bonds, both of which share their electrons equally between the bonded elements.
What is hydrophobicity and hydrophilicity?
Materials with a special affinity for water — those it spreads across, maximizing contact — are known as hydrophilic. Those that naturally repel water, causing droplets to form, are known as hydrophobic.
Which molecule is nonpolar?
Examples of homonuclear nonpolar molecules are oxygen (O2), nitrogen (N 2 ), and ozone (O 3 ). Other nonpolar molecules include carbon dioxide (CO 2) and the organic molecules methane (CH 4 ), toluene, and gasoline. Most carbon compounds are nonpolar.
What is hydrophobic material?
Hydrophobic materials are known as non-polar materials with a low affinity to water, which makes them water repelling. A contact angle of less than 90° indicates hydrophilic interaction where as an angle greater than 90° indicates a hydrophobic interaction. … Superhydrophilic surface has a contact angle of less than 5°.
Which molecule is polar?
Polar molecules are those that possess regions of positive and negative charge. Water is an example of a polar material. The type of bonds it has, when coupled with its shape, gives one end of the molecule a slight positive charge (the hydrogen end) and the other a slight negative charge (the oxygen end).
What is non-polar and polar?
In simple terms, polar means oppositely charged, and non-polar means equally charged. Covalent bonds can be polar or non-polar. To understand the difference between polar and non-polar bonds, it is essential to comprehend electronegativity.
Which group is the best hydrophilic group?
The best hydrophilic group is found in OH- group. so use is some hydroxyl group solution.
What reaction does anhydride react with?
1) Don't forget to consider that the anhydride will react with the water to form carboxylic acid groups.
What pH should an amide surface have?
It is interesting also that amide-surface should have even smaller contact ange, both operating at pH ca. 7 and even at pH < 7.
Is hydrolysis rate higher than ester?
The hydrolysis rate is lower than the one of the acid chloride but higher than that of an ester. It will hydrolyze probably already in humid air.
Is benzene acyl halide hydrolyzed?
I sent you my expected reaction in my experiment environment. The benzene acyl halide is hydrolyzed and benzene carboxylic acid is produced. Then reaction of produced carboxylic acid and remained acyl halide is expected that leads to anhydride product.