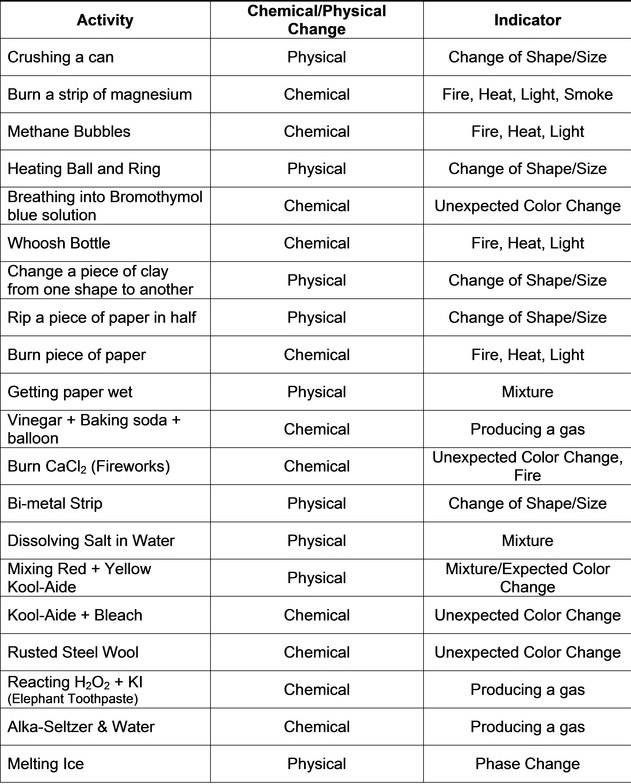
What would cause a chemical change in water?
The Properties of Chemical Changes Are
- The molecular composition of the changes in the reactant (s) as they form new substances that are totally different from the parent compounds.
- A change in temperature is also noticed in a chemical reaction as the molecules break into atoms or groups of atoms to form new compounds. ...
- A chemical change is permanent. ...
Can water undergo a chemical change?
Yes, for example, water can melt, a physical change, but it can also bond chemically with other compounds. Correct. Matter can undergo both physical AND chemical changes. A physical change is when the state of matter is changed, or even the appearance of it. A chemical change changes the substance and how it bonds.
Is water breaking down a chemical change?
Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen: 2 H2O → 2 H2 + O2 Efficient and economical water splitting would be a technological breakthrough that could underpin a hydrogen economy, based on green hydrogen. A version of water splitting occurs in photosynthesis, but hydrogen is not produced. The reverse of water splitting is the basis of the hydrogen fuel cell.
Is water being heated up a chemical change?
Similarly, when a material changes phase, it only changes physically; the substance is still the same. Think about ice melting into water, and then water being heated up and turning into steam. The chemical structure of water is the same whether it is a solid (ice), liquid, or gas (steam).
What is physical change?
What happens to matter when it changes?
What are some examples of physical changes?
What is chemical property?
Is ice still water?
Is texture a physical change?
Is water a physical or chemical change?
See more
About this website

Is making water a physical or chemical change?
chemical changeThe synthesis (forming) of water (H2O) from hydrogen gas (H2) and oxygen gas (O2) is another example of chemical change. A simplified diagram of this reaction is shown in Figure 13.3. The chemical bonds between O in O2 and between H in H2 are broken and new bonds between H and O (to form H2O) are formed.
Why is water a physical change?
Physical changes are processes in which a material changes its physical appearance but not its basic identity. The evaporation of water is a physical change. When water evaporates, it changes from the liquid state to the gas state, but it is still water; it has not changed into any other substance.
Is water a physical change?
Physical changes alter only the size, shape, form or matter state of a material. Water boiling, melting ice, tearing paper, freezing water and crushing a can are all examples of physical changes. On the other hand, chemical changes are a bit different. In a chemical change, a new substance is formed.
Is boiling water a chemical change?
No, boiling water is not a chemical change because the chemical composition of water remains unchanged on boiling. Only the physical state of water changes from liquid to water vapors during the boiling of water. The water vapors formed on boiling have the same molecular structure as that of liquid water ie; H2O.
Is water evaporating a chemical change?
Melting, evaporation and condensation are examples of physical change, or change of state, and are distinct from changes that cause new materials to form through a chemical reaction.
Can water cause a chemical change?
For instance, when an electric current is passed through water (H2O), it can be broken down into hydrogen and oxygen or H2 + O2. In this example, water is broken down into its two elements. The result is a chemical change because the starting and ending molecules are different.
Which is not a chemical change?
The correct answer is Freezing of water. Freezing is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point. Freezing of water is not a chemical change as ice when melt changes back to water showing the physical change.
Which is a chemical change?
A chemical change occurs when one substance is transformed into one or more new products via a chemical reaction. In a chemical change, the number and type of atoms remain constant, but their arrangement is altered. Most chemical changes are not reversible, except via another chemical reaction.
What are the two types of changes that matter undergoes?
4.9/5 (1,830 Views . 38 Votes) Matter can undergo changes of two basic types: physical changes and chemical changes. The evaporation of water is a physical change. When water evaporates, it changes from the liquid state to the gas state, but it is still water; it has not changed into any other substance. Click to see full answer.
What happens when water evaporates?
Water, when it boils and evaporates, changes to steam. Water will evaporate at ordinary temperatures, going into the air as a vapour (gas). Additionally, what type of reaction is water evaporating? Evaporation, is a physical process whereby water in li An Exothermic Reaction will have an Exothermic change, whereby energy (In the form of heat) ...
Is evaporation a chemical change?
Just so, is evaporation not a chemical change? Explanation: It is a physical change because it is going from the liquid phase to the gas phase. It is not a chemical change because it is still made of two hydrogen atoms and an oxygen atom.
Is melting a chemical or physical change?
While chemical change is when you have a substance that gets transformed into 1 or more different substances. Thus, melting is a physical change.
What is the difference between a physical and chemical reaction?
Remember that the difference between a physical reaction and chemical reaction is that a chemical reaction cannot be easily reversed, if at all.
What are the physical changes?
Types of some physical changes are texture, shape, temperature, and a change in the state of matter. A change in the texture of a substance is a change in the way it feels. For instance, a block of wood may feel rough when you run your finger across it but rubbing the wood with sandpaper smooths the surface so it no longer feels rough. The wood itself has not changed during sanding to become a new material, only the texture of the surface changed. A piece of metal may be heated in a fire until it glows, but the metal is the same material before heating and after cooling. Similarly, when a material changes phase, it only changes physically; the substance is still the same. Think about ice melting into water, and then water being heated up and turning into steam. The chemical structure of water is the same whether it is a solid (ice), liquid, or gas (steam).
What happens to a chemical reaction?
A chemical change occurs when the composition of a substance is changed, which requires the breaking and forming of chemical bonds during a chemical reaction. This results in the rearranging of atoms in substances to form the products of a chemical reaction, which are brand new molecules that cannot be easily reverted back to their original state.
How does temperature change in a chemical reaction?
In a chemical alteration, the temperature change occurs as a result of the breaking or formation of chemical bonds. When the chemical bonds of the reactants are broken, sometimes excess energy is released, causing heat to be discharged, and leading to an increase in temperature. Alternatively, a reaction may require energy from the environment in order to take place, causing heat to be absorbed, and leading to a decrease in temperature. Burning wood is an example of a reaction that releases excess energy as heat. A chemical cold pack in a first aid kit is an example of a chemical reaction that absorbs heat energy resulting in cooling.
What is the chemical reaction between vinegar and baking soda?
A common chemical reaction is the mixing of vinegar and baking soda. When these two household chemicals are mixed together , it immediately starts bubbling and foaming. The bubbles are a release of carbon dioxide gas, a product of the chemical reaction between the baking soda and vinegar.
How to tell if a chemical reaction has taken place?
To help determine whether there has been a reaction, chemists consider the basic indicators that a reaction has occurred, such as a change in temperature, a change in color, the development of an odor, the formation of a precipitate, or the formation of a gas.
What is an example of a color change signaling a chemical reaction?
An example of a color change signaling a chemical reaction can be observed when iron reacts with oxygen to produce iron oxide, such as when an iron nail is left outside, and it develops a reddish-brown rust.
What is a physical change?
A physical change is a temporary and easily reversible change in which the physical properties (e.g., physical state , shape, size, appearance, density etc.) of a substance change. Some physical changes are: glowing of an electric bulb, sublimation of iodine, melting of wax, melting of sulphur, evaporation of water and other liquids, formation of dew, crystallization of salt, freezing of liquids, drying of wet clothes, bending of a glass tube, etc.
What are some examples of chemical changes?
Some Common Examples of Chemical Changes 1 Cooking of food 2 Food turning bad after a few days 3 Curdling of milk 4 Fading the colours of clothes 5 Ripening of fruits 6 Lightening of a matchstick by striking match head with the side of Matchbox 7 Digestion of food within our bodies 8 Respiration by humans, plants and animals
What is the change of state of wax and butter?
Melting of wax and butter is the change of state.
What happens when ice melts?
When ice is heated, it melts and turns into water. When the water is heated further, it turns into steam. The steam condenses and becomes water when it cools. The water forms into ice as it cools further.
What is it called when a change is temporary?
The changes are temporary in nature and can be reversed. Such changes are called physical changes.
Is a physical change temporary?
In all physical changes, the change is temporary and reversible. No new substance is formed.
Does change happen by itself?
Change does not happen by itself. A cause always accompanies a change in a substance. Ice, for example, does not melt to generate water on its own. Ice must be heated to melt and turn into water. Thus, ‘heat’ is the cause of the change of state of ice from solid to liquid.
What is chemical change?
Chemical change is any change that results in the formation of new chemical substances. At the molecular level, chemical change involves making or breaking of bonds between atoms. These changes are chemical:
When you change something, does the substance change?
When you physically change something, the actual substance does not change. For example Gold is still Gold even if you melt it down into a liquid,
What happens when water molecules are forced away from each other?
boiling water (water molecules are forced away from each other when the liquid changes to vapor, but the molecules are still H2O.)
What happens when density changes in water?
What happens when density changes in water is that the atomic structure changed. As water is cooled, the atoms vibrate a bit less as they have less energy. This continues to happen until water freezes when it then forms a crystalline structure that is much different than the typical "Mickey mouse” looking atomic arrangement of liquid water. While each individual molecule is still one hydrogen, 2 oxygen, the oxygen now form a tetrahedral that repeats over all of the ice that allows for more free space. This increase in free space yields less Hs and Os to be present in a given volume, which means the density is lessened (keep in mind that nothing has a mass of 0, and if the things that filled the "open space” were allowed to join this structure, and were heavier than h2o, then the density would in fact be more when this crystalline structure formed)
What are some examples of physical changes?
Another example of a physical change is if you heat something up, say an iron bar. It will be hotter and will expand by a predictable amount but it’s still an iron bar.
Does water change its chemical composition?
Good question! Water doesn't change chemical composition, this is most obvious when the composition doesn't even change when it's phase changes (ice, water, vapor) while all of these are in fact water (h2o) the densities are very different. A cube if ice sits nicely at the top of your glass of water so clearly it's density is very different
Is color a chemical change?
Color. The changing of color of a substance is not necessarily an indicator of a chemical change. For example, changing the color of a metal does not change its physical properties. However, in a chemical reaction, a color change is usually an indicator that a reaction is occurring.
What is physical change?
Physical and Chemical Changes. A physical change is any change NOT involving a change in the substance's chemical identity. There is no effect on the chemical identity of the substance. For example, water remains water, no matter if it solid, liquid or gas.
What happens to matter when it changes?
During a physical change, the form of matter may change, but not its identity. There are some important things to remember about physical changes in matter: Arrangement of particles When a physical change occurs, the compounds may re-arrange themselves, but the bonds in between the atoms will not break.
What are some examples of physical changes?
Examples of chemical changes are burning, cooking, rusting, and rotting. Examples of physical changes are boiling, melting, freezing, and shredding.
What is chemical property?
A chemical property is any of a material's properties that becomes evident during, or after, a chemical reaction; that is, any quality that can be established only by changing a substance's chemical identity. They can also be useful to identify an unknown substance or to separate or purify it from other substances.
Is ice still water?
If liquid water is boiled, it is still water; likewise frozen water, or ice, is still water. Melting, boiling, or freezing simply by the application of a change in temperature are examples of physical changes, because they do not affect the internal composition of the item or items involved.
Is texture a physical change?
Common Physical Changes Texture. The texture of a substance can differ with a physical change. Color. The changing of color of a substance is not necessarily an indicator of a chemical change. Temperature. Although we cannot see temperature change, unless if a change of state is occurring, it is a physical change. Shape.
Is water a physical or chemical change?
The evaporation of water is a physical change. When water evaporates, it changes from the liquid state to the gas state, but it is still water; it has not changed into any other substance. For example, hydrogen burning in air undergoes a chemical change in which it is converted to water.