What would happen if water were a non polar molecule?
What would happen if water was non-polar? The reason water is a liquid at room temperature is that the water molecules stick to each other with hydrogen bonds and make it difficult for any one water molecule to break free and evaporate (become a gas). If water was non-polar, it could not form hydrogen bonds and therefore would be a gas at room temperature.
Why is water considered a polar molecule?
What are the three main properties of water?
- Polarity. A water molecule is slightly charged on both ends.
- Cohesion. Hydrogen bonds hold water molecules together, as seen in the picture above.
- Adhesion.
- High Specific Heat.
What cause water to be a polar molecule?
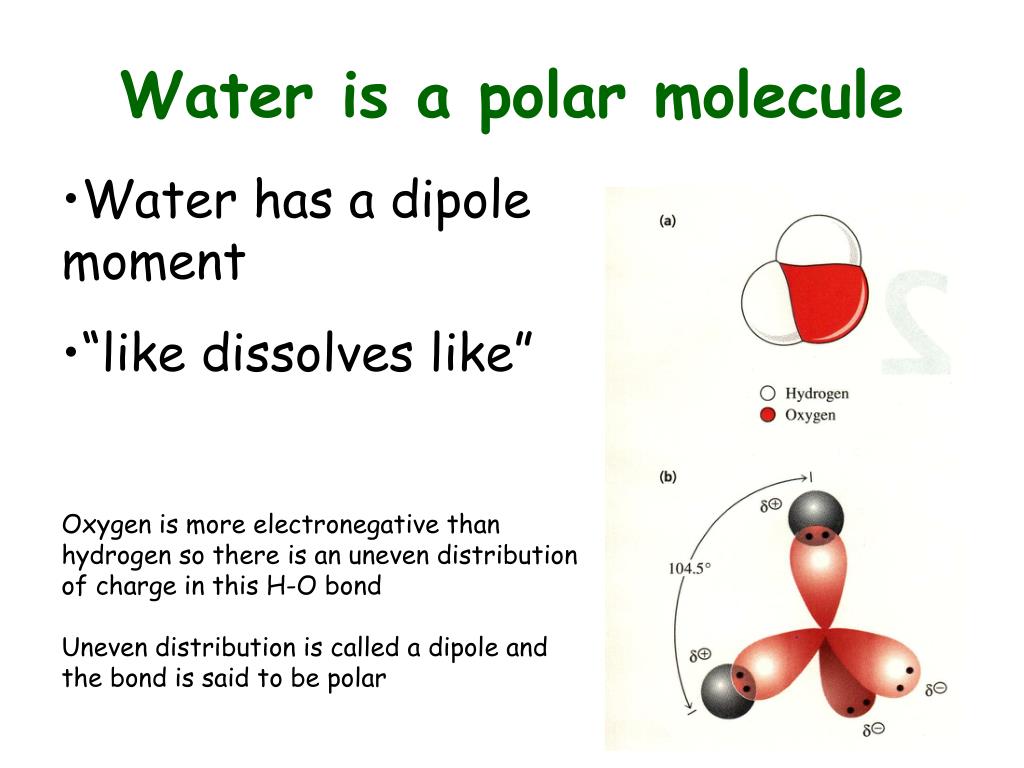
Water molecule is Polar due to its shape. Water has two positively charged hydrogen atoms and one negatively charged oxygen atom bonded covalently in a single water molecule. The hydrogen atoms have one electron in each of them and the oxygen has 4 electron pairs. Two electron pairs of oxygen form covalent bonding with two electrons of hydrogen.
What molecules would be polar or non polar?
Non-polar molecules The molecules having positively charged end and negatively charged end due to the difference in the charges of atoms present in the molecules are polar molecules. The molecules that do not have such separation of electric charges are nonpolar. Most of the polar molecules have an asymmetric or uneven distribution of electrons.

Is H2O a polar or nonpolar molecule?
Water (H2O) is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. This is an example of polar covalent chemical bonding.
Why does a water molecule have polarity?
Water (H2O) is a polar molecule because the electrons of the hydrogen atoms get "pulled" towards the electrons of the oxygen atom. This makes a region of positive charge on the hydrogen atoms and the negative charge on the other end of the molecule, which is the oxygen atom. This also allows for hydrogen bonding.
Which molecule is nonpolar?
Nonpolar Molecule Examples Examples of homonuclear nonpolar molecules are oxygen (O2), nitrogen (N2), and ozone (O3). Other nonpolar molecules include carbon dioxide (CO2) and the organic molecules methane (CH4), toluene, and gasoline. Most carbon compounds are nonpolar.
Why is water called a polar solvent?
Water interacts differently with charged and polar substances than with nonpolar substances because of the polarity of its own molecules. Water molecules are polar, with partial positive charges on the hydrogens, a partial negative charge on the oxygen, and a bent overall structure.
Why is water a polar molecule quizlet?
A water molecule is polar because there is an uneven distribution of electrons between the oxygen and hydrogen atoms. The negative pole is between the hydrogen atoms. Each molecule of water can form multiple hydrogen bonds with other water molecules.
What causes a polar bond?
A polar bond is a type of covalent bond. A bond between two or more atoms is polar if the atoms have significantly different electronegativities (>0.4). Polar bonds do not share electrons equally, meaning the negative charge from the electrons is not evenly distributed in the molecule. This causes a dipole moment.
Why is water polar?
The polarity of water molecules shows many unique physical properties. One of the most particular reasons of water being a polar molecule is its bent shape. The bond angle between the O-H bonds in the H2O molecule is around 104.5 degrees.
Why are molecules polar?
Due to the formation of partial ionic charges, molecules become polar molecules with one side being charged highly positive and other side being highly negative. Molecules formed using an equal covalent bond to share electrons, with no ionic charge and symmetrical sharing of electrons are called nonpolar molecules.
What type of bond is a single, double, or triple bond?
Covalent bonds can be a single, double or triple bond on the basis of the number of electrons shared among the atoms. Covalent bonds can form polar or non-polar molecules. Polar bonds are formed when two molecules are created using a covalent bond.
How do ionic bonds form?
Ionic bonds are formed when atoms of opposite charge and signs attract each other to create neutralized molecules. Covalent bonds form in a condition where atoms can share electrons to create molecules. Covalent bonds can be a single, double or triple bond on the basis of the number of electrons shared among the atoms.
What is the polarity of a molecule?
The polarity of a molecule majorly depends on its constituent atoms and their arrangement around the central atom. Polar molecules tend to attract water molecules, particularly through a Hydrogen bond.
What are the three states of matter that are standardized by the polarity and hydrogen bonds?
It is the only known compound that exists in all three states of matter, namely solid, liquid, and gaseous form even in the standard environment.
Which molecule is more negative than the zone around the hydrogen atoms?
The highly electronegative oxygen molecule pulls in electrons or negative charge to it, making the district around the oxygen more negative than the zones around the two hydrogen atoms. The electrically positive segments of the hydrogen molecules are bent with the two filled orbitals of the oxygen.
What makes a molecule polar?
The polarity of a molecule is related to the shifting of electrons in a particular direction. This, in turn, depends on the polarity of the bonds present in the molecule, as these bonds also contain electrons.
How does polarity depend on atoms?
The polarity of a molecule depends not only on its constituent atoms, but also on how they are arranged around the central atom, i.e. the spatial arrangement of those atoms. To understand this better, let’s discuss the topic in more detail.
Why is oxygen polar?
Since oxygen is more electronegative than hydrogen, the electron density shifts towards oxygen in both of these bonds, thereby making the region around the oxygen more negative than the areas around the two hydrogen atoms. This is why the water molecule becomes polar! References. University of Hawaii System. The University of Arizona, Tucson, ...
Why is carbonyl polar?
Carbonyl compounds are polar because the carbonyl carbon is slightly positive. Thus, shouldn’t carbon dioxide, which contains a positive carbon and two partially negative oxygens, be polar? Well, carbon dioxide consists of two oxygen atoms attached to a carbon atom. Oxygen atoms are far more electronegative than carbon atoms, and as such, ...
How many valence electrons does water have?
The hydrogen atoms only consist of one electron in their shell, whereas the oxygen atom has 6 valence electrons.
What happens to electron density in polar bonds?
All in all, you could say that the electron density of a polar bond accumulates towards one end of the bond, which results in that end possessing a slight negative charge, while the other end has a slight positive charge. This makes a molecule polar.
How many pairs of electrons are there in an oxygen atom?
Notice the 2 lone pairs of electrons on the oxygen atom in water.
Why H2O is a polar molecule?
H 2 O is made up of polar O-H bonds. Due to the difference in electronegativities of O and H atoms, O strongly attracts the shared electron cloud from each O-H bond.
Between H2S and H2O, which one is polar?
Both H 2 S and H 2 O are polar molecules. But H 2 O is certainly more polar than H 2 S. The two molecules are quite similar in shape and bonding.
Why H2O bond angle is greater than the bond angle in an H2S molecule?
The H-O-H bond angle is 104.5 ° while the H-S-H bond angle is 92.1 °. This is because an S atom is larger in size than an O atom. The electronegativity of S is less than that of O.
How do polar water molecules get attracted to each other?
Water molecules get attracted to each other using their oppositely charged O δ- and H δ+ ends.
Explain hybridization in H2O?
If we talk about the hybridization present in a water molecule then the central oxygen atom is sp 3 hybridized. The electronic configuration of Oxygen is 1s 2 2s 2 2p 4.
Is a soap molecule polar or nonpolar?
Soap molecules have both properties of non-polar and polar at opposite ends of the molecule. The oil is a pure hydrocarbon so it is non-polar. The non-polar hydrocarbon tail of the soap dissolves into the oil.
Is gas polar or nonpolar?
Gasoline is not soluble in water. Gasoline is a complex mixture of non-polar compounds such as long chained hydrocarbons etc. Water is a polar molecule. The general solubility rule is that “like dissolves like”, meaning polar dissolves polar and non-polar dissolves non-polar.
Is lamp oil polar or nonpolar?
The water and lamp oil layers do not mix because lamp oil is a hydrocarbon, which is nonpolar. Water, on the other hand, is a polar molecule.
Is kerosene polar or non polar?
3. Refer to section B. Kerosene is a nonpolar compound, water is a polar compound and isopropyl alcohol is a covalent molecule with a polar part and a nonpolar part.
Is h20 a polar or nonpolar molecule?
The Nitrogen atom is more electronegative and therefore the valence electrons are near them more often. This makes it more negative. The Hydrogen atoms at the bottom of the structure are then more positive. Therefore, H2O is a polar molecule.
Is glucose a polar molecule?
The structure of glucose is as such: as can be seen, there are multiple instances of -OH groups which are highly electronegative and draw electrons away from the carbon molecules. This leads to a skewed charge distribution. It is thus polar.
Is acetone polar or non polar?
Acetone is another molecular material with both polar and nonpolar characteristics. Here acetone has been added to water (left tube) and carbon tetrachloride (right tube). As you can see, it has mixed with both the polar water molecules and with the nonpolar carbontetrachloride molecules.
Why is water polar?
Water ( H 2 O) is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. This is an example of polar covalent chemical bonding.
Why is the shape of a molecule nonpolar?
The reason the shape of the molecule isn't linear and nonpolar (e.g., like CO 2) is because of the difference in electronegativity between hydrogen and oxygen.
What is the difference between oxygen and hydrogen?
A large difference between electronegativity values is seen with ionic bonds. Hydrogen and oxygen are both acting as nonmetals under ordinary conditions, but oxygen is quite a bit more electronegative than hydrogen, ...
How does water interact with other molecules?
The shape of each water molecule influences the way it interacts with other water molecules and with other substances. Water acts as a polar solvent because it can be attracted to either the positive or negative electrical charge on a solute. The slight negative charge near the oxygen atom attracts nearby hydrogen atoms from water or positive-charged regions of other molecules. The slightly positive hydrogen side of each water molecule attracts other oxygen atoms and negatively-charged regions of other molecules. The hydrogen bond between the hydrogen of one water molecule and oxygen of another holds water together and gives it interesting properties, yet hydrogen bonds are not as strong as covalent bonds. While the water molecules are attracted to each other via hydrogen bonding, about 20% of them are free at any given time to interact with other chemical species. This interaction is called hydration or dissolving.
What is the bent conformation of water?
The bent conformation is a balance between attraction and repulsion. Remember that even though the covalent bond between each hydrogen and oxygen in water is polar, a water molecule is an electrically neutral molecule overall. Each water molecule has 10 protons and 10 electrons, for a net charge of 0.
Which atoms are flexed away from the two filled orbitals of the oxygen molecule?
The electrically positive portions of the molecule (the hydrogen atoms) are flexed away from the two filled orbitals of the oxygen.
Is water a polar molecule?
Anne Marie Helmenstine, Ph.D. Updated May 06, 2019. Water is a polar molecule and also acts as a polar solvent. When a chemical species is said to be "polar," this means that the positive and negative electrical charges are unevenly distributed. The positive charge comes from the atomic nucleus, while the electrons supply the negative charge.
Why is a molecule polar?
A molecule is polar if there’s a significant difference in the electronegativity charges between elements. The bonds don’t cancel each other out and are asymmetrical. A nonpolar molecule has no separation of electric charges or difference in electronegativity. The bonds cancel each other out, are symmetrical, and there’s no lone electron pair.
How do you know if a molecule is nonpolar or polar?
How Do I Know If A Molecule is Nonpolar Or Polar? Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments.
How many electrons does a molecule have?
Molecules have an odd number of electrons (e.g., NO). Molecules in one or more atoms have more than eight electrons (e.g., SF6). Molecules with less than eight electrons; an example is the BF3. There are molecules with a polar bond, but the molecular geometry is symmetrical. As a result, they are nonpolar molecules by nature (examples: CO2, SO3).
What is polarity in biology?
Polarity is one of the properties of a compound related to other properties such as boiling and melting point, solubility, and molecular interactions between molecules. Polar and nonpolar molecules differ significantly. Here are the steps to help you determine if a molecule is polar or nonpolar. Table of Contents [ Show]
What is the electronegativity difference between bonded atoms?
The electronegativity difference between bonded atoms is less than 0.4. Polar bonds: The electronegativity difference is greater than 0.4 between bonded atoms. There is at least one side of the molecule with more negative or positive charge than another side.1. 3.
Do nonpolar and polar molecules have electronegativity?
Nonpolar and polar molecules exhibit some degree of electronegativity difference between bonded atoms. [1]
Do polar and nonpolar molecules have the same electron pair?
Polar molecules have an unshared electron pair while the nonpolar molecule doesn’t. If you look at pictures of polar and nonpolar molecules, they vary in symmetry.