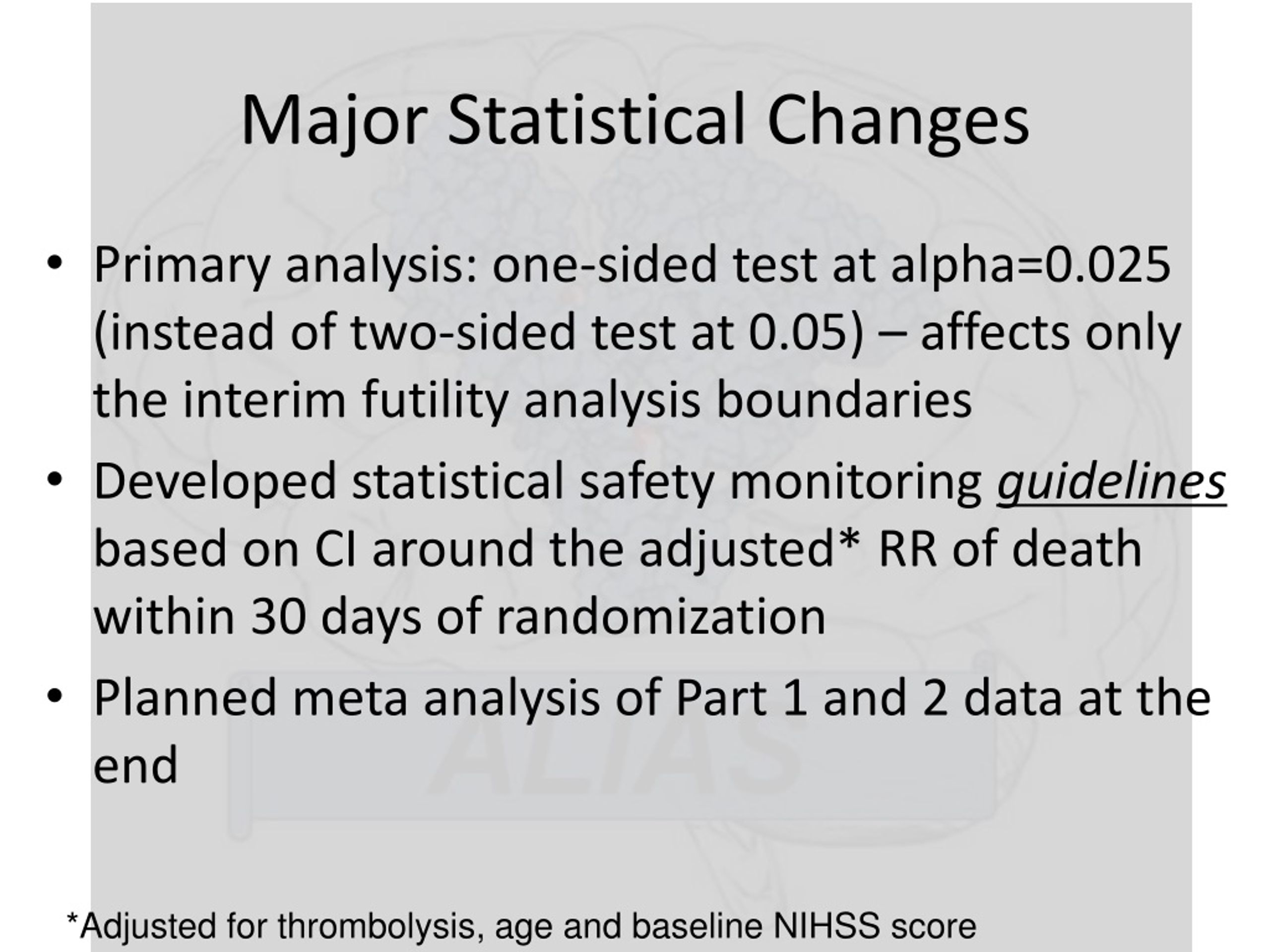
If the study nurse or coordinator is directly involved with the handling of subjects/subject data, it is generally recommended to include him/her on the 1572. It is clear we need better guidance on these 1572 questions and/or better instructions for completing the 1572 FDA regulations use the terms "investigator" and "sub-investigator":
Full Answer
Is there a guide for filling out and maintaining the 1572?
Many common mistakes are made when filling out and maintaining the 1572 form, so the hope is that this guide will be useful to new sites, clinical research coordinators (CRCs), clinical research associates (CRAs), and other clinical research professionals.
Who should be listed on the 1572 for a clinical trial?
In cases when the sponsor selects a PI who is not a physician, a qualified sub- investigator (physician) must be listed on the 1572 for the trial to make all medical-related decisions. {4} Q: What types of laboratories should be listed in this section?
What is the statement of investigator Form FDA 1572?
The Statement of Investigator, Form FDA 1572 (1572), is an agreement signed by the investigator to provide certain information to the sponsor and assure that he/she will
Do you need a 1572 for a foreign clinical study?
If a foreign clinical study is conducted under an IND, then all FDA IND regulations, including the requirement to obtain a signed 1572, must be met. If a study is conducted outside of the U.S. and is not conducted under an IND, then the investigator need not sign a 1572.
See more

Do study coordinators need to be on the 1572?
Note: If a research coordinator is performing critical study functions and collecting and evaluating some study data, the coordinator should be listed on the 1572. If the research coordinator is only transcribing data and maintaining study files, the coordinator does not need to be listed.
Who should be included on 1572?
A new 1572 is required when an investigator is participating in a new protocol that is added to an active IND and when the Principal Investigator of an ongoing study changes, when a Sub-Investigator is added, and when there is a change of location in which the study is being conducted.
What section of the 1572 is required for sub investigators?
Section 6 is provided for delivering names of individuals listed as sub-investigators.
Who signs FDA form 1572?
When new investigators are assigned to a clinical investigation under an investigational new drug application (IND), the sponsor completes and signs a Form 1572 before allowing the investigator to get involved in the clinical investigation.
Do all studies need a 1572?
Under FDA regulations, a 1572 is only required for studies of investigational drugs and biologics conducted under an IND. It is not required for studies that are not done under an IND and is not applicable to investigational device studies.
Who should be concerned by FDA Form 1572 and why?
Under the US regulations, all US clinical trial sites must be subject to the US IND and all US clinical trial investigators must sign Form 1572. All study sites (foreign or US) that are listed on the IND must comply with all applicable US regulations and the Principle Investigator (foreign or US) must sign Form 1572.
What is on a 1572?
A form that must be filed by an investigator running a clinical trial to study a new drug or agent. The investigator agrees to follow the U.S. Food and Drug Administration (FDA) Code of Federal Regulations for the clinical trial.
Do you submit 1572 to IRB?
IRBs can place any additional requirement upon a clinical research trial that they deem necessary. If they require that the initial Form 1572 and subsequent updates be sent to the IRB, then this absolutely becomes a GCP requirement.
Who can be a sub investigator?
If the PI of a clinical trial is not a physician, a qualified dentist or physician must be listed as a Sub-Investigator. Other Sub-Is can include board-certified physicians, fellows, residents, or non-physicians. Those listed as Sub-Is must make direct and significant contributions to the data.
When Should 1572 be submitted?
Note: a Form FDA 1572 must be submitted to the FDA within 30 days of the investigator being added. When changing any site information: IRB, laboratory, or clinical site.
How do I fill out a FDA 1572?
Field 1: NAME OF AND ADDRESS OF INVESTIGATOR.Field 2: EDUCATION, TRAINING, AND EXPERIENCE THAT QUALIFY THE INVESTIGATOR AS AN.EXPERT IN THE CLINCIAL INVESTIGATION OF THE DRUG FOR THE USE UNDER INVESTIGATION.Field 3: NAME AND ADDRESS OF ANY MEDICAL SCHOOL, HOSPITAL, OR OTHER RESEARCH.More items...
Can FDA Form 1572 be signed electronically?
For documents created electronically on a file system, signatures may be obtained electronically if a mechanism is available to the signer. For example, an FDA 1572 Statement of Investigator form can be filled out within Adobe and signed using an available digital certificate outside of any document management system.
What is the FDA form 3455?
applicants may submit a single FORM FDA 3455, with attachments clearly identifying all clinical investigators with information to disclose and, for each investigator, identifying the study, the specific details of their financial interests and arrangements and the steps taken to minimize the potential for bias.
When Should 1572 be updated?
If there are any changes to the information on the signed and dated 1572, the investigator must document the changes in the study records and update the sponsor. The sponsor must update the IND accordingly. However, the 1572 does not need to be edited and a new one is not required.
What is the key responsibility of the investigator in a clinical trial?
A clinical investigator's primary responsibility is to conduct research that contributes to generalizable knowledge while protecting the rights and welfare of human participants.
Which monitoring visit would not include an inventory of investigational agents quizlet?
Which monitoring visit would NOT include an inventory of investigational agents? Termination site visit.
How long does it take to get a 1572?
In cases when a new site is added or of replacement of an investigator at an existing site, a 1572 must be submitted to the FDA within a 30-day window of the site’s/investigator’s addition/replacement.
What is the Form FDA 1572 (Statement of Investigator)?
The Statement of Investigator (Form FDA 1572) is a form that is required to be filled for clinical trials involving investigational drugs or biologics. Through this form, the PI provides specific information to the sponsor, including his/her qualifications and information about the clinical site, in aim of assuring conduct of the clinical trial according to FDA regulations and guidelines. {1} By signing the 1572 form, the PI is making a legal commitment to adhere to FDA expectations by:
How to update Section 1 of the PI?
In any case when the PI is replaced with another investigator, Section 1 must be updated by filling out a new 1572 and supporting all required documentation listed in Section 2 in this form. {2}
What is the code of Federal Regulations for reporting adverse events?
Reporting all adverse events and serious adverse events to the sponsor that occur during the conduct of the trial in accordance with Title 21 CFR 312.68 in the Code of Federal Regulations.
What to do if PI name changed in 1572?
Most sponsors require that if the PI listed in the current 1572 has his/her name changed for any reason (e.g., marital status), the document should be If a sub-investigator has a name change, then in most cases sponsors ask for the form to be updated or a note to file provided explaining the name discrepancy from an audit and Good Clinical Practice (GCP) perspective.
When to use the continuation page?
Use the Continuation Page if additional space is needed.
Does FDA 1572 have an expiration date?
Office of Management and Budget’s clearance of the form as meeting the requirements of the Paperwork Reduction Act. Despite the fact the form carries an expiration date, there is no need to provide a new form after the new version with the latest expiration date has been released.
What is the purpose of the 1572?
The 1572 has two purposes: 1) to provide the sponsor with information about the investigator's qualifications and the clinical site that will enable the sponsor to establish and document that the investigator is qualified and the site is an appropriate location at which to conduct the study, and;
What is the FDA regulation for subinvestigators?
FDA's regulation at 21 CFR 3 12.3(b) states: "In the event an investigation is conducted by a team of individuals, the investigator is the responsible leader of the team. 'Subinvestigator' includes any other individual member of that team." 21 CFR 3 12.53(c)(l)(viii) requires the investigator to provide "A list of the names of the subinvestigators (e.g., research fellows, residents) who will be assisting the investigator in the conduct of the investigation(s)."
Is there a co-investigator in block #l?
Co-investigators should not be listed in Block #l. The term co-investigator is not defined in FDA regulations. As commonly used, the term is meant to indicate that each co-investigator is fully responsible for fulfilling all of the obligations of an investigator as identified in 21 CFR 3 12.60. Thus under 2 1 CFR 3 12.3(b), each co-investigator is an investigator, and as such must sign a separate 1572. It is acceptable to have more than one investigator at a particular site. This is distinct from a subinvestigator (see #30) whose role in the study is more limited.
Do you have to sign a 1572?
No. There is no need to prepare and sign a new 1572 when the OMB expiration date has been reached. The date on the form refers to the Office of Management and Budget's time frame during which FDA may collect information contained in this form.
Can a foreign study be conducted under an IND?
No. A sponsor may choose, but is not required, to conduct a foreign clinical study under an IND. When a foreign clinical study is conducted under an IND, all FDA IND requirements must be met unless waived (see Question 11 below). When the foreign clinical study is not conducted under an IND, the sponsor must ensure that this study complies with 21 CFR 3 12.120 "Foreign clinical studies not conducted under an IND" if the sponsor intends to submit the study to FDA to support clinical investigations conducted in the United States and/or marketing approval. An application based solely on foreign clinical study data must meet criteria listed in 2 1 CFR 3 14.106.
Is a 1572 required for a drug study?
No. Under the regulations, a 1572 is only required for studies of investigational drugs and biologics conducted under an IND. It is not required for studies that are not done under an IND, and is not applicable to investigational device studies. Sponsors of device studies must obtain a signed agreement (containing information similar to that requested on the 1572) from each participating investigator, per 21 CFR 8 12.43(c)
Does the FDA require a 1572 form?
No. Although the sponsor is required to collect the 1572 from the investigator, FDA does not require the form to be submitted to the agency. Many sponsors submit the 1572 to FDA, however, because it collects, in one place, information that must be submitted to FDA under 21 CFR 3 12.23(a)(6)(iii)(b).
Who does not need to be listed on delegation logs?
Personnel considered “fee for service” who are in supporting roles such as couriers, drivers, receptionists and administrative staff do not need to be listed on the delegation logs.
What is the role of the investigator in the DoD log?
The investigator is responsible for identifying key personnel to be included in the DoD Log. Key personnel can be described as clinical staff who are responsible for the following: study coordination, data collection, data management, recruitment, enrollment, informed consent, and regulatory compliance.
What is delegation of duties?
By definition, delegation of duties is entrusting someone else to do parts of your job. In clinical research, this means investigators can delegate study-related tasks to their staff members to perform on their behalf, but they never relinquish responsibility for those tasks and their outcomes.
Do you need to use the same initials on a DoD log?
Use the full first and last name and ensure the names always appear in the same order. Middle name is not required except when there are 2 people with the same initials. Use the same initials on the DoD log as you would use when initialing source documents.
Do you need to list administrative staff on the DoD log?
Personnel in supportive roles such as couriers, drivers, receptionists, and administrative staff do not need to be listed on the DoD log.
Do clinical staff need to be listed under assessment?
Any clinical staff involved in the assessment must be listed under this category. If the term is confusing or sites prefer different categories, please use the “Other” sections to add more categories.
Can you include DAIDS in a DoD log?
Yes, as long as all the required elements from the DAIDS template DoD log are included.

What Is The Form FDA 1572 (Statement of Investigator)?
When Must The Form FDA 1572 Be signed?
- According to U.S. regulations, the Form FDA 1572 is required to be collected from all PIs for studies being conducted under an Investigational New Drug (IND) application, which would include clinical studies of an investigational product or biologic, excluding device-related clinical trials (which require a similar form called an “investigator agreement” to be filled out under an In…
When Must The Form Be Updated Or A New One completed?
- In cases when a new site is added or of replacement of an investigator at an existing site, a 1572 must be submitted to the FDA within a 30-day window of the site’s/investigator’s addition/replacem...
- Another case when a 1572 should be updated is when any site information is changed, such as the IRB or laboratory affiliated with that site.{3}
- In cases when a new site is added or of replacement of an investigator at an existing site, a 1572 must be submitted to the FDA within a 30-day window of the site’s/investigator’s addition/replacem...
- Another case when a 1572 should be updated is when any site information is changed, such as the IRB or laboratory affiliated with that site.{3}
- Most sponsors require that if the PI listed in the current 1572 has his/her name changed for any reason (e.g., marital status), the document should be If a sub-investigator has a name change, then...
Dissecting The Form FDA 1572 For Principal Investigators and Sub-Investigators
- Sections 1 and 2 Important notes to keep in mind when filling Sections 1 and 2 include: 1. Section 1: The name of the PI must match his/her legal name as it appears on legal documents, certificates, or qualifications (e.g., birth certificates, marriage certificate, medical licenses, or other titles). In cases when a co-investigator is assigned, the...
Common Mistakes Identified in Audits
- Submission of incorrectly completed forms.
- Missing submission of requested documents in Section 2, especially when study personnel have been added to the 1572 or in cases when the original PI has been replaced.
- Failure to submit updated 1572 forms to both IRBs and sponsors.
- Site not having CVs for all study personnel listed on the 1572 form.
Form FDA 1572 Expiration Date
- The most recent version of the Form FDA 1572 can be obtained from www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM074728.pdf. In cases when a Form FDA 1572 is being collected shortly before a new version is released, sponsors can use the current version to obtain signed agreements from clinical investigators participating in t…
Conclusion
- For new clinical research professionals entering the field or in need of a refresher to their current knowledge, this paper was written as a guide to all study site staff, including CRCs, CRAs, PIs, and sub-investigators. It is very important to understand the many regulatory documents used in clinical trials—what they mean and how to fill out and maintain them properly. As there may be …
References
- TransCelerate Biopharma Inc. Information and guidance sheet for the completion of the Statement of Investigator Form. (Form FDA 1572). http://www.transceleratebiopharmainc.com/wp-content/uploads/20...
- U.S. Food and Drug Administration. Form FDA 1572 (Statement of Investigator) (OMB No. 0910-0014, expiration date February 28, 2019). https://www.fda.gov/downloads/AboutFDA/R…
- TransCelerate Biopharma Inc. Information and guidance sheet for the completion of the Statement of Investigator Form. (Form FDA 1572). http://www.transceleratebiopharmainc.com/wp-content/uploads/20...
- U.S. Food and Drug Administration. Form FDA 1572 (Statement of Investigator) (OMB No. 0910-0014, expiration date February 28, 2019). https://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms...
- http://regardd.org/drugs/initial-ind-submission
- U.S. Food and Drug Administration. Information Sheet Guidance for Sponsors, Clinical Investigators, and IRBs. Frequently Asked Questions—Statement of Investigator (Form FDA 1572). https://www.fda.g...
Resource
- Sather S. 2019. The CRC’s Guide to Coordinating Clinical Research (Fourth Edition). CenterWatch. Boston, Mass. https://store.centerwatch.com/p-604-the-crcs-guide-to-coordinating-clinical-research-fourth-edition.aspx The author of this article is a clinical research professional who wishes to remain anonymous.