Why are some alcohols soluble in water?
Why are some alcohols soluble in water? because carbon atoms combine with OH- to form hydrogen bonds with water. Alcohols with 4 or less carbons as more soluble
What alcohol is insoluble in water?
are non polar: they will resist solubility in water (Like dissolves like!). The non polar “tail” in hexanol is much longer than in ethanol, and this makes hexanol insoluble in water . You see that the solubility decreases as the non-polar chain becomes longer.
What compounds are soluble and insoluble in water?
- All nitrates are soluble in water, so Zn (NO 3) 2 is soluble.
- All bromides are soluble in water, except those combined with Pb 2+, so PbBr 2 is insoluble.
- All phosphates are insoluble, so Sr 3 (PO 4) 2 is insoluble.
What solutes are soluble in water?
table salt,baking powder,soaps,detergents,sugar,alcohols all tend to be more soluble in hot water,since their dissolution in water is endothermic in nature. you may even find many organic compounds like heavier alcohols,carboxylic acids to get dissolve in hot water which are almost insoluble in cold water.
Which alcohols is most soluble in water?
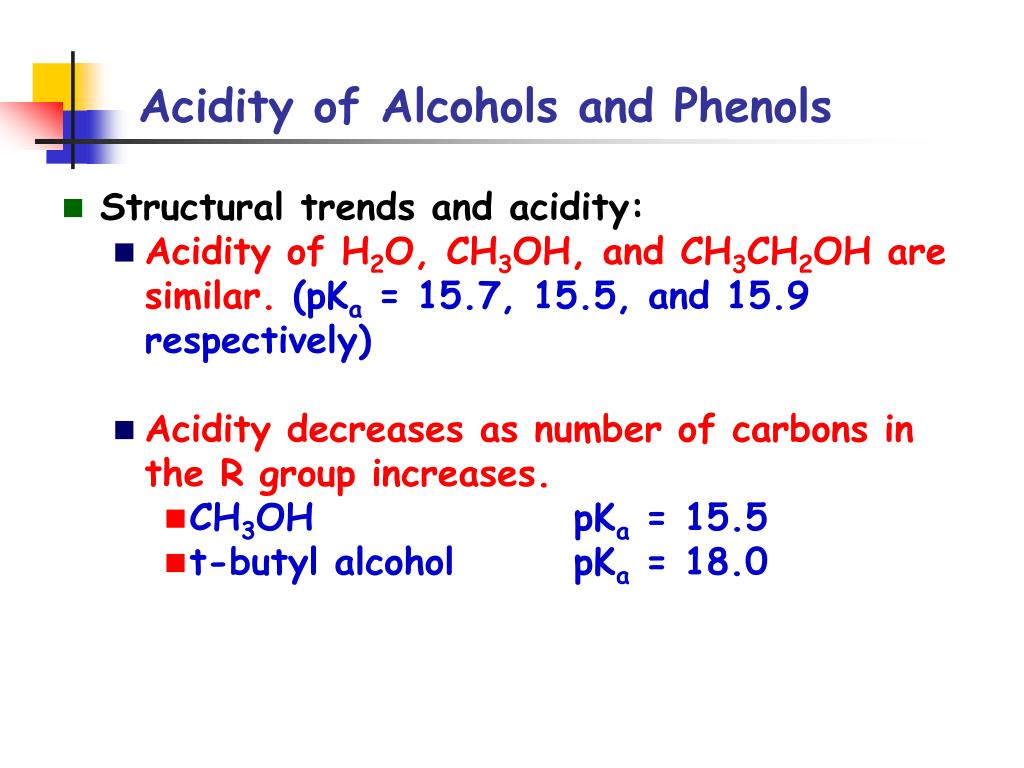
Solution : Solubility increases as the branching increases. Therefore t-butyl alcohol is most soluble in water.
Are all alcohols equally soluble in water?
Smaller alcohol molecules tend to be more soluble than larger alcohol molecules. While small alcohol molecules like methanol and ethanol are completely miscible in water, alcohol molecules larger than n-octanol (C8H17OH) tend not to be soluble in water at all.
What alcohols is least soluble in water?
So the alcohol that should be the least soluble in water is pentan-1-ol.
Why alcohols are not soluble in water?
Lower alcohols are highly soluble in water but higher alcohols are insoluble or less soluble in water due to their molecular weight.
Is vodka miscible in water?
Because of the strength of the attraction of the OH group, first three alcohols (methanol, ethanol and propanol) are completely miscible. They dissolve in water in any amount.
Which alcohol is highly soluble?
Lower alcohols are more soluble in water than higher alcohols.
Is ethanol soluble in water?
Substances composed of small polar molecules, such as acetone and ethanol, are usually soluble in water.
Which is most soluble in water?
Sugar is the most soluble chemical in water among the substances listed.Sugar has six hydroxyl groups. ... Sugar is made from sucrose, and its molecule is more complex and bigger than the ions in the salt.When energy is applied to polar sucrose molecules, intermolecular interactions with polar water molecules develop.More items...
Are all alcohols soluble?
Thus, whereas the hydrocarbons are insoluble in water, alcohols with one to three carbon atoms are completely soluble.
Is butanol soluble in water?
n-Butanol has limited miscibility in water; however, it is easily soluble in regular solvents such as ethers, alcohol, glycols and hydrocarbons. This solvent is very flammable, with a flashpoint of around 35° C.
Which is least soluble in water?
C2H6 being non-polar is least soluble in water (Given the polar nature of H2O).
What are the weakest alcohols?
In this list you will find the most common drinks and their alcoholic degrees.Low-alcohol beer: 0.05 – 1.2%Kefir: 0.2 – 2.0%Kombucha: 0.5 – 1.5%Chicha: 1.0 – 11% (generally varies from 1 to 6%)Beer: 2.0 – 12%Cider: 2.0 – 12%Mead: 8 – 16%.Wine: 5.5 – 16% (generally 12.5 – 14.5%)More items...•
Is ethanol less soluble in water?
Ethanol has an overall polarity that allows it to make hydrogen bonds to water easily, allowing solubility. Methanol, ethanol and propanol have just less enough hydrocarbon chains to make the -OH to H2O bond possible (i.e. soluble).
Why are Alcohols Soluble in Water?
Water molecules are polar and form intermolecular hydrogen bonds amongst them. They are also capable of forming hydrogen bonding with other similar molecules.
Which Alcohol is most Soluble in Water?
As discussed in the earlier section the solubility of alcohols in water decreases as the number of carbon atoms increases.
Solubility of Primary, Secondary, and Tertiary Alcohols in water
However, from butanol onwards, the solubility of alcohol in water is greatly affected and the bulky linear chain alcohol molecules tend to form separate layers from water when the two are dissolved together.
Solubility Chart for Alcohols
The alcohols consist of a polar group viz. hydroxyl functional group (-OH group) which is hydrophilic and a non-polar group viz. alkyl group which is hydrophobic.
Factors Affecting the Solubility of Alcohols
The solubility is the property of a substance to dissolve in another substance. The substance here can be solid, liquid, or gas.
What are the three alcohols that are miscible?
Because of the strength of the attraction of the OH group, first three alcohols (methanol, ethanol and propanol) are completely miscible. They dissolve in water in any amount.
What is the chemical formula for alcohol?
Each alcohol consists of a carbon chain (always nonpolar) and a OH group (which is polar). For ethanol for example the chemical formula looks lie this: C 2 H 5 OH.
Can alcohol dissolve in water?
They dissolve in water in any amount. Starting with the four-carbon butanol the solubility of alcohols is starting to decrease. After the 7-carbon heptanol, alcohols are considered immiscible. The alcohol solublity in water can be observed below.
What causes alcohol to break apart?
The positive and negative dipoles in the molecules align, tugging at one another and causing the alcohol molecules to break apart from one another in the water and dissolve.
What forces pull ethanol molecules apart?
Dipole-dipole and hydrogen bond intermolecular forces pull the ethanol molecules apart from one another.
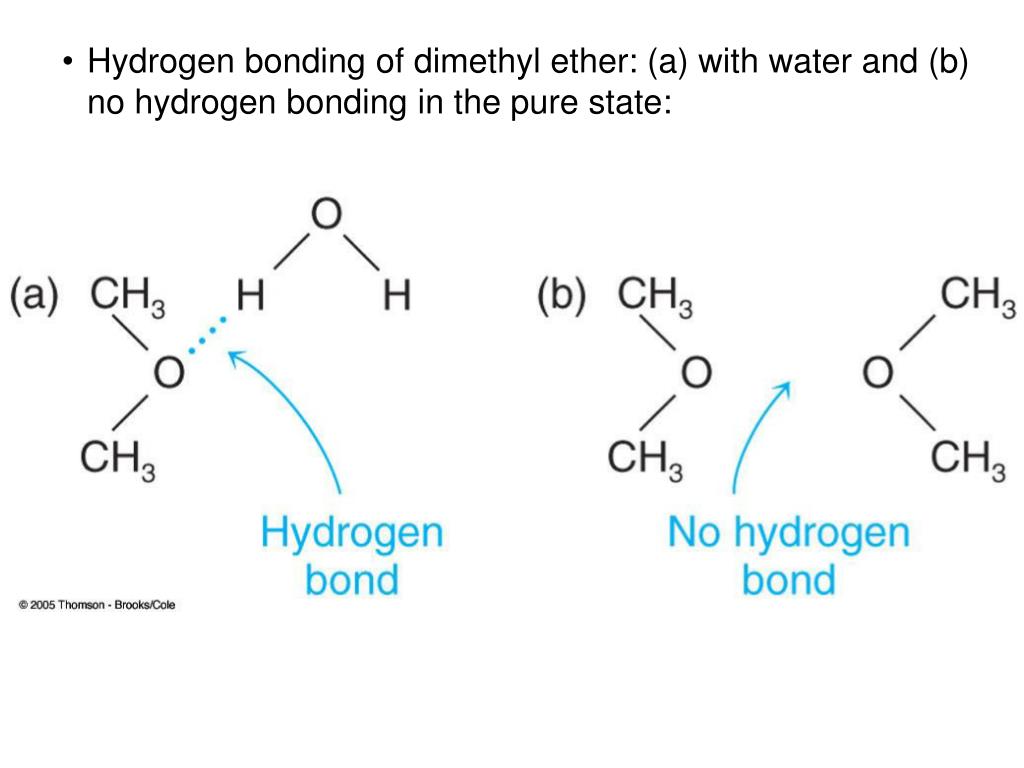
Why does alcohol form a hydrogen bond with water?
Due to high electronegativity difference oxygen gained partial negative charge and hydrogen gained partial positive charge. As a result, the negative pole of alcohol is attracted by positive pole of water and the positive pole of alcohol is attracted by negative pole of water i.e. alcohol can form hydrogen bond with water.
How does the length of a hydrocarbon chain affect the solubility of water?
As the length of the hydrocarbon chain increases, the solubility in water decreases. With four carbon in the hydrocarbon chain and higher, the decrease in solubility becomes visible as the mixture forms two immiscible layers of liquid.
Why can't ethers dissolve in water?
So ethers cannot dissolve in water because the two types on bonding do not do well together. Alcohol also form hydrogen bonds. However, alcohols are made of a hydroxy group and an alkyl group.
Is alcohol a generic term?
Sugar and alcohol are generic terms that describe classes of compounds. For example, glucose and fructose are also sugars; methanol and isopropyl alcohol are also alcohols. Most substances have a measurable level of solubility in any given solvent, at some temperature.
Is sugar an alcohol?
I am assuming that when you say sugar you mean sucrose and when you say alcohol you mean ethyl alcohol. Sugar and alcohol are generic terms that describe classes of compounds. For example, glucose and fructose are also sugars; methanol and isopropyl alcohol are also alcohols.
Is thymolphthalein water soluble?
Note that there is a problem with the statement that thymolphthalein is not water soluble. This compound is used as an indicator in acid-base tritrations (usually added from a solution in ethanol, 1 drop or ~0.1 mL, so just a dash of ethanol). For thymolphthalein to act as an indicator it must be in solution.
Is ethanol a water mixture?
In a 50% - 50% ethanol - water mixture, there is no such distinction. Anyway, liquids are often miscible if they are chemically similar. Both ethanol and water have a chemical formula of the form R-OH. In water, R is Hydrogen, in ethanol it is the ethyl group CH₃CH₂-. 5.1K views.

Why Are Alcohols Soluble in Water?
Which Alcohol Is Most Soluble in Water?
- As discussed in the earlier section the solubility of alcohols in water decreases as the number of carbon atoms increases. Methanol has the smallest alkyl group out of all the alcohols due to which it is most soluble in water. However, this is to be noted that both ethanol and methanol are completely soluble in water at room temperature. So, how do...
Solubility of Primary, Secondary, and Tertiary Alcohols in Water
- Amid primary alcohols the order of solubility is Methanol > Ethanol > Propanol However, from butanol onwards, the solubility of alcohol in water is greatly affected and the bulky linear chain alcohol molecules tend to form separate layers from water when the two are dissolved together. On the other hand in the case of branched-chain alcohols, the bulky groups are still soluble and t…
Solubility Chart For Alcohols
- The alcohols consist of a polar group viz. hydroxyl functional group (-OH group) which is hydrophilic and a non-polar group viz. alkyl group which is hydrophobic. Therefore, the solubility of alcohol in water is determined by the strength of these two forces acting against each other. The solubility is measured in mol/100g of H2O at 1atm and 25 degrees Celcius.
Factors Affecting The Solubility of Alcohols
- The solubility is the property of a substance to dissolve in another substance. The substance here can be solid, liquid, or gas. It depends upon the physical and chemical properties of the substance. The substances with similar kinds of intermolecular forces of attraction tend to be better soluble with each other. In the case of alcohols the solubility is dependent on a number o…
Conclusion
- • Alcohols are soluble in water due to the formation of hydrogen bonding between alcohol and water molecules and the polar nature of both liquids. • The hydroxyl group, owing to its hydrophilic nature and capacity of hydrogen bond formation, is responsible for the solubility of alcohols in water. • Amongst primary alcohols, the order of solubility is Methanol > Ethanol > Propanol. • In t…