An alloy is a mixture of chemical elements, which forms an impure substance (admixture
Mixture
In chemistry, a mixture is a material system made up of two or more different substances which are mixed but are not combined chemically. A mixture refers to the physical combination of two or more substances on which the identities are retained and are mixed in the form of solutions, suspensions, and colloids.
What are 10 examples of alloys?
Examples of alloys
- Steel. This alloy is essential for the construction industry since it is used to make beams or supports for pouring concrete or concrete. ...
- Brass. ...
- Bronze. ...
- Stainless steel. ...
- Amalgam. ...
- Duralumin. ...
- Pewter. ...
- White gold. ...
- Magnalium. ...
- Wood’s Metal. ...
What are examples of alloys and their uses?
Uses of Alloys. 1. Ornaments. White gold, yellow gold, rose gold are various alloys of gold often used in the making of jewelry and accessories. Other than this, Britannia silver and sterling silver are the alloys of silver that are used to make ornaments and other decorative showpieces. 2. Soldering.
What alloys are commonly used?
Examples of Alloys and Their Components
- The use of alloys in gold-based ornaments with copper is well known for ages and is mainly used for making the ornaments durable and wearable. ...
- The alloy brass is used for many home decorative items and is composed of copper and zinc. ...
- The alloy of bronze is used for making statues and medals as well as home decorates. ...
What are common uses of alloys?
- Pipe fittings like elbows, plugs, and couplings
- Taps, faucets, and other fixtures
- Valve bodies

What is a alloys Class 9?
Alloy is a metal made by combining two or more metallic elements, especially to give greater strength or resistance to corrosion. They are homogeneous mixture of two or more substances. An example of an alloy is the tin in the mixture of copper and tin that makes bronze.
What are alloys write with example?
It is not uncommon for alloys to have greater strength and hardness when compared to pure metals. An example of an alloy is red gold, which is produced by alloying copper and gold together. Another important alloy of gold is white gold, which is produced by alloying silver and gold together.
What are alloys and its types?
There are two main types of alloys. These are called substitution alloys and interstitial alloys. In substitution alloys, the atoms of the original metal are literally replaced with atoms that have roughly the same size from another material. Brass, for example, is an example of a substitution alloy of copper and zinc.
What are alloys 10 answers?
An alloy is a homogeneous mixture of two or more metals or a metal and a non-metal.
Why are alloys used?
Almost all metals are used as alloys—that is, mixtures of several elements—because these have properties superior to pure metals. Alloying is done for many reasons, typically to increase strength, increase corrosion resistance, or reduce costs.
What are 3 types of alloy?
Here are some of the most popular alloys and their applications.Stainless Steel Alloys. Stainless steel is an alloy comprised of iron and carbon. ... Aluminum Alloys. ... Bronze Alloys. ... Nickel Alloys.
What are the 5 alloys?
List of five important alloys:- 1. Steel Alloy 2. Copper Alloys 3. Aluminum Alloys 4....Magnesium Alloys.Steel Alloy: ... Copper Alloys: ... Aluminum Alloys: ... Nickel Alloys: ... Magnesium Alloys:
How are alloys named?
Some alloys are named after their primary constituent. For example, the "silver" used in jewelry and the "aluminum" used as a structural building material are actually alloys. Alloys of gold are rated on a scale of carats—for instance, 14-carat gold is 58 percent gold.
What are alloys give Example Class 9?
Solution : A metallic substance made by mixing and fusing two or more metals, or a metal and a non-metal, to obtain desirable qualities such as hardness, lightness and strength is known as an alloy. E.g. : Brass, bronze, steel, etc.
What is an alloy class 8?
Answer: Alloy is a mixture (or a solid solution) of two or more metals, or one or more metals along with a non-metal. Alloys are generally made by combining two metals in their molten state.
What are some examples of alloy metals?
Examples of alloys include red gold (gold and copper) white gold (gold and silver), sterling silver (silver and copper), steel or silicon steel (iron with non-metallic carbon or silicon respectively), solder, brass, pewter, duralumin, bronze, and amalgams.
What are the 5 alloys?
List of five important alloys:- 1. Steel Alloy 2. Copper Alloys 3. Aluminum Alloys 4....Magnesium Alloys.Steel Alloy: ... Copper Alloys: ... Aluminum Alloys: ... Nickel Alloys: ... Magnesium Alloys:
1. What do you mean by Alloy?
An alloy is an amalgamation of a metal or metals and other elements. Several metals or elements can be added to the existing metal for enhancing th...
2. How are Alloys formed?
An alloy of a metal is formed when it is combined with one or more elements. The most common and one of the oldest methods to alloy a metal is carr...
3. What are the Needs of Making an Alloy?
The needs for making alloys are:Improve the Hardness of Metal - Alloy making increases the hardness of metal. For ex : when we convert soft gold in...
4. Write a Short Note on the Following :(i) Alnico (ii) Solders
(i) Alnico - Alnico is also known as aluminum nickel-cobalt steel. This alloy is made up of Aluminum (10 to 12%), Nickel (15 to 20%) and Cobalt (40...
5. What is the Heating Treatment of Steel? Explain the Processes Involved in Heat Treatment of Steel...
In heat treatment of steel, steel is heated or cooled according to a strictly determined temperature schedule. The heat treatment helps in: Alterin...
What is an alloy?
Alloy, metallic substance composed of two or more elements, as either a compound or a solution. The components of alloys are ordinarily themselves metals, though carbon, a nonmetal, is an essential constituent of steel. Almost all metals are used as alloys—that is, mixtures of several elements—because these have properties superior to pure metals. ...
What is an alloy in chemistry?
Alloy, metallic substance composed of two or more elements, as either a compound or a solution.
What is a fusible metal?
The term fusible metals, or fusible alloys, denotes a group of alloys that have melting points below that of tin (232° C, 449° F). Most of these substances are mixtures of metals that by themselves have low melting points, such as tin, bismuth, and lead.
Why is deoxidation important?
Deoxidation is also important before alloying steel with easy oxidizable metals such as chromium, titanium, and vanadium, in order to minimize losses and improve process control. Metals that do not oxidize readily, such as nickel, cobalt, molybdenum, and copper, can be added in the…
Why are alloys added to steel?
steel: Alloying. Alloy ing elements are added to steels in order to improve specific properties such as strength, wear, and corrosion resistance. Although theories of alloying have been developed, most commercial alloy steels have been developed by an experimental approach with occasional inspired guesses.
What are the elements that make up steel?
The principal alloying elements for steel are chromium, nickel, manganese, molybdenum, silicon, tungsten, vanadium, and boron. Alloy steels have a wide range of special properties, such as hardness, toughness, corrosion resistance, magnetizability, and ductility.
Why is deoxidation important before alloying?
Deoxidation is also important before alloying steel with easy oxidizable metals such as chromium, titanium, and vanadium, in order to minimize losses and improve process control. Metals that do not oxidize readily, such as nickel, cobalt, molybdenum, and copper, can be added in the…. steel: Alloying.
How are Alloys Designed?
Alloys are made by mixing a metal with other elements (usu ally more metals). This “mixing” can be done by melting the elements together (casting, induction melting, or vacuum arc melting).
Why are Alloys Useful?
By alloying–adding new elements to a pure metal–you can achieve new properties. For example, suppose you mixed lead and tin. Doing this will result in a lower melting point than either pure metal!
What is superalloys in metallurgy?
Superalloys are another pinnacle of metallurgy. Superalloys are like micro-scale composites. They have a matrix (solid solution) phase, and a precipitate (intermetallic) phase. Like steels, modern superalloys may have 10-20 alloying elements.
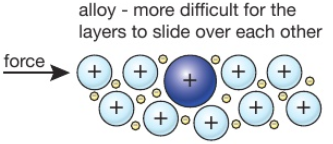
Why are alloys stronger than pure metals?
Why alloys are stronger than pure metals. A material’s strength is its resistance to deformation. Metals move by dislocations, so a stronger metal means it’s more difficult to slide atoms past each other.
Why do atoms arrange randomly?
The atoms arrange randomly because it maximizes entropy. Entropy is basically chaos, and the universe always tries to increase entropy. That’s the 2nd law of thermodynamics. This “entropic driving force” can actually change what kind of alloy results.
Why are alloys important?
Metals are useful for their conductivity, reflectivity, formability, and warning signs that they will break . They are relatively cheap to mine and manufacture.
What is the process of combining metals to make alloys?
A more expensive way of making alloys is called powder metallurgy, which squishes metal particles together and fuses them in a process called “sintering.” Powder metallurgy has many advantages over regular melting because you can combine elements with dissimilar melting points and make sure the mixture is homogeneous. However, it’s not easy to make metal powder!
What is an alloy?
Ans. An alloy is an amalgamation of a metal or metals and other elements. Several metals or elements can be added to the existing metal for enhancing their properties. For example, pure aluminium and pure copper are relatively softer metals.
What is an alloy in chemistry?
Let us now learn what alloy means in chemistry. An alloy refers to a combination of two or more metals, or a metal combined with one or more elements. The resulting alloy has different properties than the original elements altogether, like increased strength and hardness.
What are the constituents of an alloy?
Constituents of Alloy. Now that you have learned about what is the meaning of alloy, let us look at what are the constituents of alloy. An alloy consists of two or more elements, either as a compound or a solution. The components of alloys are generally metals.
What is an alloy? What are some examples?
However, the properties which alloys exhibit are different than the individual properties of these elements. When you compare it to the pure metals, alloys are stronger and harder. Alloy examples include red gold which is made by combining gold and copper together. Another example of alloy includes white gold which is an amalgamation of silver and gold. Today, we will learn about what is an alloy in chemistry, alloy definition and constituents of alloy.
What are some examples of alloys?
When you compare it to the pure metals, alloys are stronger and harder. Alloy examples include red gold which is made by combining gold and copper together. Another example of alloy includes white gold which is an amalgamation of silver and gold. Today, we will learn about what is an alloy in chemistry, alloy definition and constituents of alloy.
How is an alloy formed?
An alloy of a metal is formed when it is combined with one or more elements. The most common and one of the oldest methods to alloy a metal is carried out by heating the metal beyond its melting point. The solutes are then dissolved into the molten liquid.
What is the difference between bronze and steel?
Since steel has higher tensile strength and affordability, it is used in the infrastructure and construction of buildings, bodies of vehicles and electrical appliances. Bronze. Bronze is known to be an alloy of tin and copper. It is commonly used in heavy tools and gears, coins, medals, trophies and even in different forms of electrical hardware.
What is an alloy made of?
Alloys are metals combined with other substances in order to make them better in some way. While some people assume the term ‘alloys’ means a mixture of metals, the reality is that alloys are materials made up of at least two different chemical elements, one of which is a metal.
Why do manufacturers use alloys?
Manufacturers like using alloys to improve their products’ durability, ability to withstand heat, and/or ability to conduct electricity. Alloys have been traditionally made by heating and melting components to make liquid forms which can be mixed together and cooled into a solid solution.
Why do airplanes have alloys?
Basically, alloys take a main metal and improve its physical properties so it’s stronger and harder and/or less malleable and less ductile. Manufacturers like using alloys to improve their products’ durability, ability to withstand heat, and/or ability to conduct electricity.
What is Eagle Alloys?
Eagle Alloys has been in the business of cutting, shaping and distributing essential materials to industrial companies such that sophisticated alloys can be utilized in hundreds of different, important applications. Call 800-237-9012 to discuss alloys currently available to meet your needs.
Is an alloy a compound or a solid?
Typically, an alloy has its main metal (also known as the parent or base metal) which represents 90 percent or more of the material and then its alloying agent (s) which can be either metal or nonmetal, present in smaller quantities. Some alloys can be compounds, but generally they’re in the form of a solid solution.
What is an alloy made of?
You might see the word alloy described as a "mixture of metals", but that's a little bit misleading because some alloys contain only one metal and it's mixed in with other substances that are nonmetals (cast iron, for example, is an alloy made of just one metal, iron, mixed with one nonmetal, carbon). The best way to think of an alloy is as a material that's made up of at least two different chemical elements, one of which is a metal. The most important metallic component of an alloy (often representing 90 percent or more of the material) is called the main metal, the parent metal, or the base metal. The other components of an alloy (which are called alloying agents ) can be either metals or nonmetals and they're present in much smaller quantities (sometimes less than 1 percent of the total). Although an alloy can sometimes be a compound (the elements it's made from are chemically bonded together), it's usually a solid solution (atoms of the elements are simply intermixed, like salt mixed with water ).
Why do people use alloys?
People make and use alloys because metals don't have exactly the right properties for a particular job. Iron is a great building material but steel (an alloy made by adding small amounts of nonmetallic carbon to iron) is stronger, harder, and rustproof. Aluminum is a very light metal but it's also very soft in its pure form. Add small amounts of the metals magnesium, manganese, and copper and you make a superb aluminum alloy called duralumin, which is strong enough to make airplanes. Alloys always show improvements over the main metal in one or more of their important physical properties (things like strength, durability, ability to conduct electricity, ability to withstand heat, and so on). Generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to pull into wires).
How do alloys behave?
Photo: It's not just the basic ingredients (the metals and other constituents) that affect the properties of an alloy; how those ingredients combine is very important too. Pouring or stirring speeds, pouring temperatures, and cooling rates are some of the factors that can affect the physical properties of alloys. Photo of brass alloy casting by Jet Lowe, courtesy of US Library of Congress, Prints & Photographs Division, Historic American Engineering Record.
How do alloys form?
Alloys can also form if the alloying agent or agents have atoms that are very much smaller than those of the main metal. In that case, the agent atoms slip in between the main metal atoms (in the gaps or "interstices"), giving what's called an interstitial alloy.
What is brass made of?
Brass, for example, is a substitution alloy based on copper in which atoms of zinc replace 10–35 percent of the atoms that would normally be in copper. Brass works as an alloy because copper and zinc are close to one another in the periodic table and have atoms of roughly similar size.
What do the black and red circles represent in an interstitial alloy?
Artwork: Substitution alloys and interstitial alloys: In these diagrams, the black circles represent the main metal and the red circles are the alloying agents.
How to make an alloy?
How can you mix together two lumps of solid metal? The traditional way of making alloys was to heat and melt the components to make liquids, mix them together, and then allow them to cool into what's called a solid solution (the solid equivalent of a solution like salt in water). An alternative way of making an alloy is to turn the components into powders, mix them together, and then fuse them with a combination of high pressure and high temperature. This technique is called powder metallurgy. A third method of making alloys is to fire beams of ions (atoms with too few or too many electrons) into the surface layer of a piece of metal. Ion implantation, as this is known, is a very precise way of making an alloy. It's probably best known as a way of making the semiconductors used in electronic circuits and computer chips. (Read more about this in our article on molecular beam epitaxy .)
What is an alloy in chemistry?
Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. An alloy is a substance made by melting two or more elements together, at least one of them metal.
What are some examples of alloys?
Examples of alloys include stainless steel, brass, bronze, white gold, 14k gold, and sterling silver. Although exceptions exist, most alloys are named for their primary or base metal, with an indication of other elements in order of mass percent.
How are alloys made?
An alloy is a substance made by melting two or more elements together, at least one of them metal. An alloy crystallizes upon cooling into a solid solution, mixture, or intermetallic compound. The components of alloys cannot be separated using a physical means. An alloy is homogeneous and retains the properties of a metal, even though it may include metalloids or nonmetals in its composition.
What is the name of the alloy of iron and carbon?
Steel: the name given to an alloy of iron with carbon, usually with other elements, such as nickel and cobalt. The other elements add a desired quality to the steel, such as hardness or tensile strength.
Why are alloys used in metals?
Alloys are used because their chemical and physical properties are superior for an application than that of the pure element components. Typical improvements include corrosion resistance, improved wear, special electrical or magnetic properties, and heat resistance.
What is the difference between 18k gold and stainless steel?
Stainless Steel: another iron alloy, which typically contains chromium, nickel, and other elements to resist rust or corrosion. 18k Gold: this is 75% gold. The other elements typically include copper, nickel, or zinc.
What are the two metals that make up meteorites?
Meteoritic Iron: While meteorites may consist of any number of materials, some are natural alloys of iron and nickel, with extraterrestrial origins. These alloys were used by ancient cultures to make weapons and tools. Amalgams: These are mercury alloys. The mercury makes the alloy much like a paste.
What is Alloy?
An alloy is a combination of one or more elements or metals melted together to result out a liquid which is allowed to solidify and produce a product called alloy .
Why We Create Alloys?
We create alloy because metal as a raw material is not suitable to certain applications to use. A metal may be soft to create other human useful products.
What is the classification of an alloy?
What is alloy Classification? Alloys are classified into two different types. These are listed below.. Ferrous Alloy: – A ferrous alloy is the alloy that contains the iron, carbon and the other elements like manganese, nickel, chromium, copper, vanadium, molybdenum, tungsten, etc.
Which is stronger, raw metal or alloy?
Alloys are more stronger than the raw metals.They are more resistible and have good magnetic properties as compared to the simple metal. In addition to this, the melting point of the alloy is lower than the melting point of the pure metals.
What is non ferrous alloy?
Non-ferrous Alloy: – A non-ferrous alloy is the alloy that contains no iron in combination of mixture.
What is an Alloy?
An alloy is a blend of two or more different metals or elements that lend its properties to the total compound. Alloys are created to enhance the properties of the metals and elements they contain. Think of an alloy as a current pro basketball super team, where the whole is comprised of strong individual parts. Alloys can actually exhibit unique properties that are different to the metals they are made from. In a nutshell, it’s combining the strengths of different metals and elements to produce a superior, solid solution.
Why are alloys better than pure metals?
There are several benefits to using alloys vs. pure metals. Alloys tend to be harder and stronger than pure metals, providing more durability. Alloys also provide top notch resistance to corrosive environments and rot, much more than a single pure metal could offer. Alloys are also used over pure metals to improve appearance, as they are lustrous and contain a better finish.
What is C863 alloy?
C863 Manganese/Bronze – A Manganese Bronze alloy that can operate under heavy loads at high speeds. It shows superior mechanical qualities and corrosion resistance.
What is Patriot Foundry?
Patriot Foundry is a leading non-ferrous casting foundry specializing in a variety of alloys. Browse our site to learn more about alloy casting or contact us today with any questions you may have on our services!
How many alloys are there?
In total, 20 alloys are highlighted, and they range from household names (i.e. bronze, sterling silver) to lesser-known metals that are crucial for industrial purposes (i.e. solder, gunmetal, magnox).
What is stainless steel?
The metal alloy – now known to the world as stainless steel – was a step forward in creating a corrosion-resistant steel that is now used in many applications ranging from medical uses to heavy industry.
Why is copper and tin important?
This alloy, made from copper and tin, was extremely useful to our ancestors because it is much stronger and harder than its component metals . Steel is another great example of an alloy that has changed the world. It is one of the most important and widely-used metals today.
How much steel is used in the world?
Today, it is the world’s most commonly used metal and most recycled material, with 1,864 million metric tons of crude steel produced in 2020.
Can you find metals on the periodic table?
Every day, you’re likely to encounter metals that cannot be found anywhere on the periodic table. You may play a brass instrument while wearing a white gold necklace – or maybe you cook with a cast iron skillet and store your leftovers in a stainless steel refrigerator. It’s likely that you know these common metal alloys by name, ...
