Which local anesthetic has the longest duration of action?
Which local anesthetic has the longest duration of action? Procaine and chloroprocaine are the shortest-acting agents (0.25-0.5 hours), followed by lidocaine, mepivacaine, and prilocaine, which have slightly longer durations of action (0.5-1.5 hours).
Is Lidocaine an amide or ester?
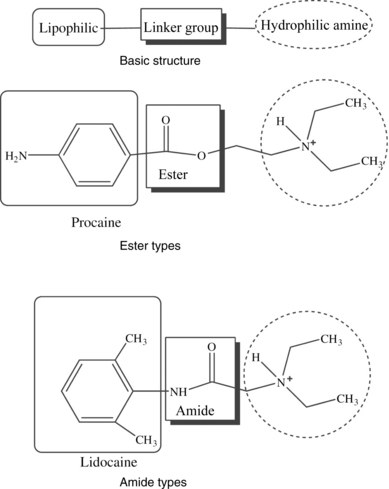
The local anesthetic agents can be divided into two chemically distinct classes: esters and amides. Local anesthetic agents in the amino ester class include procaine, chloroprocaine, and tetracaine. Amino amides used clinically include lidocaine, mepivacaine, prilocaine, bupivacaine, levobupivacaine, and ropivacaine.
Is lidocaine used for anesthesia?
Lidocaine, also known as lignocaine and sold under the brand name Xylocaine among others, is a local anesthetic of the amino amide type. It is also used to treat ventricular tachycardia. When used for local anaesthesia or in nerve blocks, lidocaine typically begins working within several minutes and lasts for half an hour to three hours.
Is Novocaine an amide or ester?
Novocain (Procaine): First synthesized in 1905, novocain (the trade name of procaine) is an ester-type local anesthetic that is able to induce a loss of sensation when injected, as opposed to oral intake which has been stated to wield therapeutic values.

What is the difference between amide and ester?
The ester and amide compounds differ in terms of their stability in solution, metabolism, and allergic potential. Amides are extremely stable in solution, while esters are unstable.
Which medicines are amide local anesthetics?
The commonly used amide LAs include lidocaine, bupivacaine, ropivacaine, mepivacaine, and outside the United States, levobupivacaine. Articaine is an amide LA used primarily in dentistry. LAs are weak bases that exist in solution in both charged and uncharged forms.
What are ester and amide local anesthetics?
Two basic classes of local anesthetics exist, the amino amides and the amino esters. Amino amides have an amide link between the intermediate chain and the aromatic end, whereas amino esters have an ester link between the intermediate chain and the aromatic end.
What are the top 3 most commonly used local anesthetics?
The commonly used drugs are amides like lignocaine, prilocaine, and bupivacaine.
What is the most common local anesthetic?
Lidocaine continues its prominence as the most widely used local anesthetic in the United States, but all of these agents have comparable efficacy.
What are the names of local Anaesthetics?
What types of local anaesthetics are there?Benzocaine.Chloroprocaine.Cocaine.Procaine.Proparacaine.Tetracaine.Amylocaine.Oxybuprocaine.
What are the 3 types of anesthesia?
Types of AnesthesiaLocal Anesthesia. Local anesthesia is an anesthetic agent given to temporarily stop the sense of pain in a particular area of the body. ... Regional Anesthesia. Regional anesthesia is used to numb only the portion of the body that will undergo the surgery. ... General Anesthesia.
What is difference between amine and amide?
No, amine and amide are not the same. Amine is an ammonia derivative in which one or more hydrogen atoms are replaced by an alkyl or aryl group, while amide is an amine derivative of carboxylic acid. A sigma bond joins a carbonyl carbon atom to a nitrogen atom bonded by hydrogen atoms or carbon atoms.
What are the four types of anesthesia?
Types of AnesthesiaGeneral Anesthesia. General anesthesia is used for major operations, such as a knee replacement or open-heart surgery, and causes you to lose consciousness.IV/Monitored Sedation. Sedation is often used for minimally invasive procedures like colonoscopies. ... Regional Anesthesia. ... Local Anesthesia.
What is the strongest local anesthetic?
In fact, tetracaine is 5 to 8 times more efficacious than cocaine and is the most potent among dental topical anesthetics.
What are the 6 types of anesthesia?
The Different Kinds of AnesthesiaThere are different types of anesthetics that may be used for your surgery. ... General Anesthesia. ... Regional Anesthesiology. ... Combined General with Epidural Anesthesia. ... Monitored Anesthesia Care with Conscious Sedation.
Which local anesthetic is least toxic?
Although lidocaine is more toxic than bupivacaine and ropivacaine, mepivacaine, which has a similar pharmacological effect to lidocaine, is the safest among clinically used local anesthetics.
How do you remember esters or amides?
A pharmacist once taught me this trick to remember how to tell whether a local anesthetic is an ester or an amide: Look at the generic name of the local anesthetic. If it contains 2 of the letter “i” then it is an amide. If it contains only 1 letter “i” then it is an ester.
Is articaine an ester or amide?
Articaine is an intermediate-potency, short-acting amide local anesthetic with a fast metabolism due to an ester group in its structure.
Is Novocaine an ester or amide?
Novocaine is a broadly used term referring to any type of local anesthetic. Technically speaking, novocaine is the same as procaine, which is an ester local anesthetic. Today, lidocaine is more commonly used than novocaine.
Why are ester anesthetic locals more prone to allergies?
Esters are associated with a higher incidence of allergic reactions, due to a p-aminobenzoic acid (PABA) metabolite. Amide agents do not undergo such metabolism. However, preservative compounds (methylparaben) used in the preparation of amide-type agents are metabolized to PABA.
What are the adverse reactions to local anesthetics?
Adverse reactions to local anesthetics are usually a reaction to epinephrine, vasovagal syncope, or overdose toxicity. Allergic reactions to local anesthetics are often attributed to additives such as metabisulfite or methylparaben. True allergic reactions to amide local anesthetics are extremely rare but have been documented. Patients with true allergy to amide local anesthetics present a challenge to the dental practitioner in providing adequate care with appropriate intraoperative pain management. Often, these patients may be treated under general anesthesia. We report a case of a 43-year-old female patient that presented to NYU Lutheran Medical Center Dental Clinic with a documented history of allergy to amide local anesthetics. This case report reviews the use of 1% diphenhydramine with 1:100,000 epinephrine as an alternative local anesthetic and reviews the relevant literature.
Is ester a local anesthetic?
Allergies to local anesthetics have been reported for ester-type local anesthetics. Hydrolysis of ester-type local anesthetics by cholinesterase results in the release of para-aminobenzoic acid, a known allergen, as a metabolite. However, recent pivotal studies of ester agents for US Food and Drug Administration approval and marketing claims report no cases of this phenomenon.3–6Amide-type local anesthetics are metabolized in the liver and are essentially free from producing allergic phenomena.4–7However, although they are rare, there have been documented cases of amide-type local anesthetic allergy.6,8
Is DPH an anesthetic?
One limitation of DPH local anesthetic is its duration of action, as it may be too short for longer procedures. Although the effectiveness of DPH for mandibular blocks was not evaluated on this patient, previous studies have shown efficacy in providing inferior alveolar nerve anesthesia15,17,20However, the volume of DPH must be limited per visit to reduce postoperative swelling and drowsiness. The administration technique with the 10-mL Becton Dickinson syringe that we used on this patient does not allow for ideal aspiration techniques, as provided by a typical dental syringe/cartridge apparatus. An aspirating Becton Dickinson syringe is available for use, if desired (Figure 4).
HOW DO AMIDE LOCAL ANESTHETICS WORK?
Amide local anesthetics are commonly used for pain control during minor surgery.
Where are amides processed?
Amide local anesthetics have an amide link in their structure and are processed in the liver.
How to manage local anesthetic emergencies?
Management of Local Anesthetic Emergencies: The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient’s state of consciousness after each local anesthetic injection. At the first sign of change, oxygen should be administered.
How does local anesthesia block nerve impulses?
Mechanism of Action: Local anesthetics block the generation and the conduction of nerve impulses presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to ...
How much 2% solution is used for caudal anesthesia?
2. Caudal and Lumbar Epidural Block: For caudal anesthesia, the initial dose is 15 to 25 mL of a 2% or 3% solution. Repeated doses may be given at 40 to 60 minute intervals.
Can you use multiple doses of anesthetic for epidural?
Local anesthetic solutions containing antimicrobial preservatives, i.e., those supplied in multiple-dose vials, should not be used for epidural or caudal anesthesia because safety has not been established with regard to intrathecal injection, either intentionally or unintentionally, of such preservatives.
Is procaine a benzoic acid?
DESCRIPTION#N# Procaine hydrochloride is benzoic acid, 4-amino-, 2- (diethylamino) ethyl ester, monohydrochloride, the ester of diethylaminoethanol and para-aminobenzoic acid.#N#The solutions are made isotonic with sodium chloride and the pH is adjusted between 3 and 5.5 with sodium hydroxide and/or hydrochloric acid.
Should local anesthesia be avoided?
DOSAGE AND ADMINISTRATION:#N#The rapid injection of a large volume of local anesthetic solution should be avoided and fractional (incremental) doses should always be used. The smallest dose and concentration required to produce the desired result should be administered.
Can you mix novocain with local anesthetic?
Mixing or the prior or intercurrent use of any local anesthetic with NOVOCAIN cannot be recommended because of insufficient data on the clinical use of such mixtures. Single-dose containers and multiple-dose containers of NOVOCAIN may be sterilized by autoclaving at 15-pound pressure, 121°C (250°F) for 15 minutes.
What are the adverse reactions to local anesthetics?
Adverse reactions to local anesthetics are usually a reaction to epinephrine, vasovagal syncope, or overdose toxicity. Allergic reactions to local anesthetics are often attributed to additives such as metabisulfite or methylparaben. True allergic reactions to amide local anesthetics are extremely rare but have been documented.
Can amide local anesthetics cause allergic reactions?
True allergic reactions to amide local anesthetics are extremely ra …. Adverse reactions to local anesthetics are usually a reaction to epinephrine, vasovagal syncope, or overdose toxicity. Allergic reactions to local anesthetics are often attributed to additives such as metabisulfite or methylparaben. True allergic reactions to amide local ...
What is the intermediate chain of anesthetics?
The intermediate chain or linkage provides a convenient basis for classification of local anesthetics, and also determines their pattern of elimination. Amides are biotransformed in the liver but esters are hydrolyzed in the bloodstream by plasma esterases. Ester local anesthetics are no longer packaged in dental cartridges and are used infrequently, with the exception of benzocaine, found in several topical anesthetic preparations. Articaine is unique in this regard. It is classified as an amide according to its intermediate linkage, but also contains an ester side chain on its aromatic ring. Hydrolysis of this side chain renders the molecule inactive, and it is therefore eliminated in a manner identical to ester anesthetics.
What are the components of local anesthesia?
The molecular structure of all local anesthetics consists of 3 components: (a) lipophilic aromatic ring, (b) intermediate ester or amide linkage, and (c) tertiary amine. Each of these components contributes distinct clinical properties to the molecule. (See Figure 1.)
How do local anesthetics affect the duration of neural blockade?
This property is expressed as the percentage of circulating drug that is protein bound and has been found to correlate with an anesthetic's affinity for protein within sodium channels as well. The greater the tendency for protein binding, the longer the anesthetic will sustain neural blockade. For example, bupivacaine exhibits 95% protein binding compared to 55% for mepivacaine, and this is credited for the difference in their duration of neural blockade.
Why do local anesthetics cause seizures?
As local anesthetics are absorbed from the injection site, their concentration in the bloodstream rises and the peripheral nervous system and central nervous system (CNS) are depressed in a dose-dependent manner. (See Figure 3.) Low serum concentrations are used clinically for suppressing cardiac arrhythmias and status seizures, but ironically, higher concentrations induce seizure activity. Convulsive seizures are the initial life-threatening consequence of local anesthetic overdose. Presumably this is due to selective depression of central inhibitory tracts, which allow excitatory tracts to run amuck. As serum concentrations continue to rise further, all pathways are inhibited, resulting in coma, respiratory arrest, and eventually cardiovascular collapse. Evidence of lidocaine toxicity may commence at concentrations >5 µg/mL, but convulsive seizures generally require concentrations >10 µg/mL.
How does lipid solubility affect anesthesia?
Greater lipid solubility of a drug not only enhances potency but also enables more rapid diffusion through cell membranes. For local anesthetics, this hastens the onset for anesthesia in isolated fibers during in vitro studies, but it must be appreciated that other factors come into play clinically. For example, inherent vasodilating properties may promote systemic absorption before the anesthetic reaches the nerve membrane. High lipid solubility may impede dispersion throughout tissue fluids and also fosters sequestration in neighboring adipose tissues or myelin sheaths. In either case, fewer numbers of molecules reach the neuronal membrane and onset is delayed. Therefore, unlike in vitro studies of isolated fibers, greater lipid solubility generally slows the onset of anesthesia in the clinical setting. Injecting higher concentrations that allow a greater number of molecules to reach the membrane and hasten onset can offset this influence. Although bupivacaine and articaine are both highly lipid soluble, the 4% concentration of articaine provides for a much faster onset.
Why are neural fibers sensitive to local anesthesia?
Also, smaller fibers are generally more susceptible, because a given volume of local anesthetic solution can more readily block the requisite number of sodium channels for impulse transmission to be entirely interrupted. For these reasons the tiny, rapid-firing autonomic fibers are most sensitive, followed by sensory fibers and finally somatic motor fibers.1,2The anesthesiologist blocking mixed spinal nerves is acutely aware of these differential sensitivities. As patients recover from spinal anesthesia they first regain voluntary motor function, then sensation returns, and finally they can micturate (autonomic control). The dentist is generally spared this consideration because the trigeminal nerve branches anesthetized for dental procedures are comprised only of small, rapid-firing sensory fibers. However, the many classes of sensory fibers also vary in their diameters and firing rates. For example, pain fibers are more sensitive than those carrying pressure and proprioception. A patient may remain disturbed by a sense of pressure despite complete anesthesia of pain fibers.
How do local anesthetics interrupt neural conduction?
Local anesthetics interrupt neural conduction by inhibiting the influx of sodium ions through channels or ionophores within neuronal membranes. Normally these channels exist in a resting state, during which sodium ions are denied entry. When the neuron is stimulated, the channel assumes an activated or open state, in which sodium ions diffuse into the cell, initiating depolarization. Following this sudden change in membrane voltage, the sodium channel assumes an inactivated state, during which further influx is denied while active transport mechanisms return sodium ions to the exterior. Following this repolarization, the channel assumes its normal resting state. An appreciation of these sodium channel states helps to explain the preferential sensitivity of local anesthetics for various classes of neuronal fibers.