Aniline Dyes
- Aniline Dyes Provide a Translucent Color to Wood Furniture. Aniline dyes are substances used to add color to wood, clothing and leather. ...
- History of Aniline Dye. ...
- Benefits of Aniline Wood Dye. ...
- Penetrating the Wood. ...
Why is aniline more acidic than ethyl amine?
In ethylamine, ethyl is electron donating group. So, more electrons are available aroudn nitrogen for donation. But in aniline, phenyl group is electron attracting group. Lone pair of electron on nitrogen is involved in delocalisation. This is the reason that ethyl amine is more basic than aniline.
What are the uses of aniline?
What are the uses of aniline?
- Anilines are used in the rubber industry for the processing of rubber chemicals and products such as car tyres, balloons, gloves, etc.
- It is used as a dyeing agent in the manufacture of clothes such as jeans, etc.
- It is employed in the production of drugs such as paracetamol, Tylenol, acetaminophen.
What does aniline smell like?
What does aniline smell like? Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. Like most volatile amines, it has the odor of rotten fish.
Which is more basic acetanilide or aniline?
aniline is more basic than acetanilide because lone pair of electron present on Nh2 + in aniline is electron donating and increase electron density at ring and resonanace takes place
See more

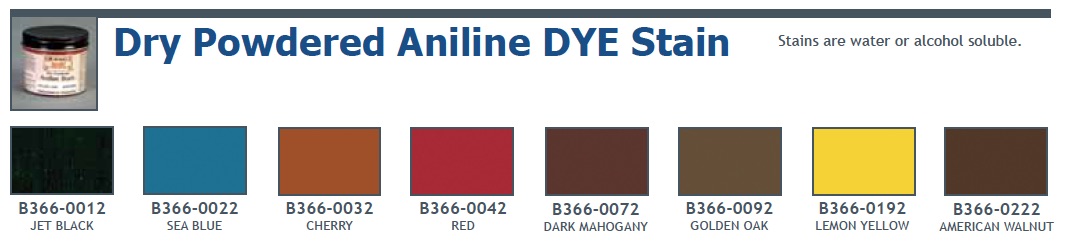
What are aniline dyes used for?
Aniline dyes were artificially produced dyes – a triumph of modern chemistry – and remain in use today. They are used commercially to dye silk, wool and other proteinaceous fibers. The following material is taken from the History page in the Chemical Dyes section of my site.
What is aniline dye made from?
Derived mainly from coal tar, synthetic dyes in general came to be known as aniline dyes, and a new chemical dyemaking industry sprang up around them. The dyes are fine powders.
Are aniline dyes natural?
Nature – the World's First Dyes Before the advent of synthetic dye compounds such as those made from aniline, fabric color came solely from nature; plants, animals, and minerals. Humans have been using dyes to color fabric for more than 2,600 years; the earliest recorded use coming from China.
What is aniline color?
Aniline appears as a yellowish to brownish oily liquid with a musty fishy odor.
Is aniline a dye?
Aniline dyes were the first synthetically produced basic dyes. The method for the production of Mauve (mauveine) was discovered in 1856 in England by William Perkin. This quickly spawned a new area of research into triphenylmethane dyes from which the second aniline based dye, Magenta (fuchsin), was discovered.
Which dye is prepared from aniline?
2-Aniline naphthol dye2-Aniline naphthol dye is made from aniline.
Who invented aniline dyes?
William Henry PerkinIn 1856, during Easter vacation from London's Royal College of Chemistry, 18-year-old William Henry Perkin (1838–1907) synthesized mauve, or aniline purple—the first commercialized synthetic dyestuff—from chemicals derived from coal tar.
When were aniline dyes invented?
1856…in England, made the first aniline dye (1856) as a result of abortive attempts to synthesize quinine, the sole antimalarial drug available at that time. About 30 years later, Ehrlich found that a synthetic dye, methylene blue, has antimalarial properties.
How long does it take for aniline dye to dry?
12 to 24 hoursAllow to dry from 12 to 24 hours, depending on atmospheric conditions, before proceeding with your finishing schedule. Water soluble dyes will not bleed into solvent based finishes.
What is aniline red dye?
Description. A monoazo red dye composed of chlorinated-p-nitroaniline. Aniline Red was discovered by Herzberg and Spengler in 1907. The bright red colorant is approved for use in drugs and cosmetics. It is also used in inks, enamels, paper, plastics, and opaque paints.
What is the common name of aniline?
aminobenzeneAniline, also known as aminobenzene or phenylamine, has 6 carbon (C) atoms, 7 hydrogen (H) atoms, and 1 nitrogen (N) atom in its chemical formula of C6H7N or C6H5NH2. Because aniline has an amino group in its structure, it is also an amine, hence it is classified as an aromatic amine.
What is an aniline dye?
Any of a group of dyes derived from aniline which is a coal-tar distillation product. Aniline dyes were the first synthetically produced basic dyes. In spite of this, they are still used in watercolors, inks and fabric dyes. See also aniline green, aniline yellow, aniline brown, and aniline black.
What is the boiling point of aniline?
Aniline appears as a yellowish to brownish oily liquid with a musty fishy odor. Melting point -6°C; boiling point 184°C; flash point 158°F. Denser than water (8.5 lb / gal) and slightly soluble in water.
Is aniline a toxic substance?
Also Know, are aniline dyes toxic? Aniline itself is a very toxic substance. The dyes we use today are much less toxic than aniline. The greatest risk of disease or injury due to modern acid dyes is by ingestion of or exposure to dye dust.
When was aniline dye invented?
The first aniline dye invented was mauveine, or purple, and it was a happenstance discovery.”. In 1856, 18-year-old chemist William Henry Perkin was doing experiments for his professor, trying to synthesize the anti-malaria drug quinine.
Who discovered the first synthetic dye?
The first synthetic dye was discovered by William Henry Perkin “ (1838-1907)” whose aniline purple, better known as mauve, was an instant success and led to further discoveries.” (230) The pairs of boots depicted in figure 4 were dyed in yellow and red, as the Victoria and Albert Museum describes: “by about 1860 chemical aniline dyes were widely ...
What color was the afternoon dress in the 1850s?
The afternoon dress shown in figure 3 was dyed in rich royal blue, a new kind of color made possible with aniline dyes in the later 1850s.
What color was Perkin's dye?
This dye created a beautiful lustrous colour that Perkin patented and which became known as ‘aniline violet’ or ‘mauveine’. Perkin’s discovery led to a revolution in synthetic colour from the late 1850s onwards.
What colors were used in the first Perkin's mauve?
The first was ‘Perkin’s mauve’, followed by a variety of shades of purples and magentas, yellows, blues, and pinks. These colours were much more intense than any available from the traditional natural dyes.”. Historian Daniel Delis Hill in the History of World Costume and Fashion (2011) gives a bit more detail:
Where did purple dye come from?
It came from a particular species of snail and took a lot of the tiny animals to create a large enough quantity of purple dye to dye an entire dress, and it faded really, really easily, which is why that color was reserved almost exclusively for royalty and members of the clergy.
Who discovered purple dye?
In The Chemical Technology of Dyeing and Printing (1948), historian Ludwig Diserens and Paul Wengraf explain how William Perkin discovered the synthetic purple: “Perkin made the experiment and there resulted in a black molasses-like mass, very far removed from the white crystal he was hoping for.
What solvent should I use for aniline dye?
But what solvent (alcohol, oil or water ) should you use in conjunction with the dye? That’s an easy call , water . Yes, water is a solvent and water-based aniline dye is what I’ve used for my entire woodworking career. Here’s why. Water is easy to find for most of us.
What is the difference between dye and stain?
It’s a dye. What’s the difference? A store-bought stain has pigment , and usually a binder to glue that pigment to your furniture , that’s suspended in a solvent . Stains sit on the surface of your work and obscure the wood’s grain (which creates what is otherwise known as a “muddy appearance.”) Dye is quite different.
How long does it take for oil based dye to dry?
Comparatively, water-based dyes can dry in about four hours. (I’ve forced more than one piece to dry more quickly with a hand-held hairdryer.)
Is water based dye easy to use?
Additionally, water-based dye is easy to use. It’s almost error proof. Compared to alcohol-based dye that dries so quickly, the water-based mixture precludes lap marks, especially if you apply the dyes as I do.
Does aniline fade in sunlight?
Water-based aniline dye is the least likely aniline dye to fade in sunlight. It does fade, but it fades at a much slower rate than either the alcohol- or oil-based dyes. You do know by now that anything placed in direct sunlight is going to fade. Additionally, water-based dye is easy to use. It’s almost error proof.
Can you use oil based dye on shellac?
If you use an oil-based dye then brush on a coat of oil or oil-based finish, there’s a chance, a good chance, that your dye will run or move as the brush strikes the surface. Ditto if you’re using alcohol-based dye and applying a coat of shellac.
Is aniline dye the same as a stain?
Dye is quite different. Dye is made up of microscopic crystals that dissolve in a solvent. As a result, dye travels wherever the solvent travels, and that’s deeper into the wood than stains. That adds depth to your finish. Aniline dye is the best method I’ve found to add color to my furniture without camouflaging the surface.
Why is aniline yellow?
Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening of the sample (to yellow or red) due to the formation of strongly colored, oxidized impurities.
What is the chemical formula for aniline?
Chemical compound. Not to be confused with the amino acid Alanine. Aniline is an organic compound with the formula C 6 H 5 N H 2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis.
What is the reaction of aniline and nitrous acid?
Aniline and its ring-substituted derivatives react with nitrous acid to form diazonium salts. Through these intermediates, aniline can be conveniently converted to -OH, -CN, or a halide via Sandmeyer reactions. This diazonium salt can also be reacted with NaNO 2 and phenol to produce a dye known as benzeneazophenol, in a process called coupling . The reaction of converting primary aromatic amine into diazonium salt is called diazotisation. In this reaction primary aromatic amine reacts with sodium nitrile and with 2 moles of HCl which is known as Ice cold mixture because the temperature use to be 0.5 °C and it forms benzene diazonium salt as major product and water and sodium chloride.
What reacts with acyl chlorides to form amides?
Aniline reacts with acyl chlorides such as acetyl chloride to give amides. The amides formed from aniline are sometimes called anilides, for example CH 3 -CO-NH-C 6 H 5 is acetanilide. At high temperatures aniline and carboxylic acids react to give the anilides.
What happens when you add bromine to aniline?
If bromine water is added to aniline, the bromine water is decolourised and a white precipitate of 2,4,6-tribromoaniline is formed . To generate the mono-substituted product, a protection with acetyl chloride is required: Aniline can react with bromine even in room temperatures in water.
What acid gives chloranil?
Hydrochloric acid and potassium chlorate give chloranil. Potassium permanganate in neutral solution oxidizes it to nitrobenzene; in alkaline solution to azobenzene, ammonia, and oxalic acid; in acid solution to aniline black. Hypochlorous acid gives 4-aminophenol and para-amino diphenylamine.
How is aniline produced?
Industrial aniline production involves two steps. First, benzene is nitrated with a concentrated mixture of nitric acid and sulfuric acid at 50 to 60 °C to yield nitrobenzene. The nitrobenzene is then hydrogenated (typically at 200–300 °C) in the presence of metal catalysts:
What is aniline dye?
Aniline dyes were artificially produced dyes – a triumph of modern chemistry – and remain in use today. They are used commercially to dye silk, wool and other proteinaceous fibers. The following material is taken from the History page in the Chemical Dyes section of my site.
What is the origin of aniline?
Aniline was first isolated from the destructive distillation of indigo, a plant used to produce blue dye, in 1826. In 1834, it was also isolated from coal tar. Further attempts by several scientists yielded aniline by different processes and, in 1841, C. J. Fritzsche gave this blue oil the name “aniline,” from the specific name of one of the indigo-yielding plants, Indigofera anil (anil being derived from the Sanskrit nīla, dark-blue, and nīlā, the indigo plant). In 1855 these variously named oils were proved to be identical , and thenceforth they took their place as one body, under the name aniline or phenylamine.
What dyes are related to triphenylmethane?
Acid dyes having structures related to triphenylmethane predominate in the milling class of dye. There are many yellow and green dyes commercially applied to fibers that are related to triphenylmethane.
What was the first dye?
The very first artificially produced chemical dye was a produced from aniline (aka phenylamine or aminobenzene), and most still use it as a precursor substance. (Then again, acetaminophen is also produced from aniline, as are polyurethane, herbicides and nanowire for use as a semiconducting electrode bridge.)
How do acid dyes bond?
Acid dyes are thought to fix to fibers by hydrogen bonding, Van der Waals forces and ionic bonding. They are normally sold as the sodium salt therefore they are anionic in solution. Animal protein fibers and synthetic nylon fibers contain many cationic sites, therefore there is an attraction of anionic dye (- charge) molecule to a cationic site (+ charge) on the fiber. The strength (fastness) of this bond is related to the “desire” of the dye to remain dissolved in water over fixation to the fiber.
What is the structure of azo dyes?
The structure of azo dyes is based on azobenzene, Ph-N=N-Ph. Although Azo dyes are a separate class of dyestuff mainly used in the dyeing of cotton (cellulose) fibers, many acid dyes have a similar structure, and most are red in color.
When was aniline first used?
Its first industrial-scale use was in the manufacture of mauveine, a purple dye discovered in 1856 by an 18 year old chemistry student, William Henry Perkin, who was trying to create artificial quinine (an anti-malarial drug). At the time of mauveine's discovery, aniline was an expensive laboratory compound, but it was soon prepared "by the ton" using a process previously discovered by Antoine Béchamp.
What is the color of aniline?
Aniline appears as a yellowish to brownish oily liquid with a musty fishy odor. Melting point -6°C; boiling point 184°C; flash point 158°F. Denser than water (8.5 lb / gal) and slightly soluble in water. Vapors heavier than air. Toxic by skin absorption and inhalation. Produces toxic oxides of nitrogen during combustion. Used to manufacture other chemicals, especially dyes, photographic chemicals, agricultural chemicals and others.
What is aniline used for?
Aniline is predominantly used as a chemical intermediate for the dye, agricultural, polymer, and rubber industries. It is also used as a solvent, and has been used as an antiknock compound for gasolines.
How much aniline is toxic?
It is classified as very toxic. Probable oral lethal dose in humans is 50-500 mg/kg for a 150 lb. person. Aniline poisoning is characterized by methemoglobin formation in the blood and resulting cyanosis or blue skin. The formation of methemoglobin interferes with the oxygen -carrying capacity of the blood. The approximate minimum lethal dose for a 150 lb. human is 10 grams. Serious poisoning may result from ingestion of 0.25 mL. People at special risk include individuals with glucose-6-phosphate -dehydrogenase deficiency and those with liver and kidney disorders, blood diseases, or a history of alcoholism. (EPA, 1998)
What happens when you mix sulfuric acid and aniline?
Mixing aniline and 96% sulfuric acid in a closed container caused the temperature and pressure to increase. Many "hydrocarbon-metal perchlorate " complexes are explosive, for example, the complexes of benzene, toluene, aniline, pyridine, and dioxane.
How many workers are exposed to aniline?
According to the 2016 TSCA Inventory Update Reporting data, 16 reporting facility estimates the number of persons reasonably likely to be exposed during the manufacturing, processing, or use of aniline in the United States may be as low as fewer than 10 workers and as high as 9,999 workers per plant; the data may be greatly underestimated due to confidential business information (CBI) or unknown values (1).
What pH buffer is used for aniline extraction?
Extraction efficiencies of the anilines /aniline and its substituted derivatives/ were determined in reagent water buffered at pH 5 (0.01 M acetate buffer), pH 7 (0.01 M phosphate buffer), and pH 11 (0.01 M phosphate buffer). Methanol solutions of the compound were spiked into one-liter volumes of the buffered media in a separatory funnel at the 10 ug/L level. Three successive extractions with 60 mL volumes of methylene chloride were performed using two-minute extraction periods. The extracts were combined and concentrated.
What is the pesticide code for aniline?
For aniline (USEPA/OPP Pesticide Code: 251400) there are 0 labels match. /SRP: Not registered for current use in the U.S., but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses./

Overview
Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame c…
Structure
In aniline, the C−N bond length is 1.41 Å, compared to 1.47 Å for cyclohexylamine, indicating partial π-bonding between N and C. The C(aryl)-NH2 distance in anilines is highly sensitive to substituent effects. This distance is 1.34 Å in 2,4,6-trinitroaniline vs 1.44 Å in 3-methylaniline.
The amine in anilines is a slightly pyramidalized molecule, with hybridization of the nitrogen somewhere between sp and sp . The nitrogen is described as having high p character. The amin…
Production
Industrial aniline production involves two steps. First, benzene is nitrated with a concentrated mixture of nitric acid and sulfuric acid at 50 to 60 °C to yield nitrobenzene. The nitrobenzene is then hydrogenated (typically at 200–300 °C) in the presence of metal catalysts:
The reduction of nitrobenzene to aniline was first performed by Nikolay Zinin in 1842, using inorganic sulfide as a reductant (Zinin reaction). The reduction of nitrobenzene to aniline was al…
Reactions
The chemistry of aniline is rich because the compound has been cheaply available for many years. Below are some classes of its reactions.
The oxidation of aniline has been heavily investigated, and can result in reactions localized at nitrogen or more commonly results in the formation of new C-N bonds. In alkaline solution, azobenzene results, whereas arsenic acid produces the violet-coloring matter violaniline. Chromi…
Uses
Aniline is predominantly used for the preparation of methylenedianiline and related compounds by condensation with formaldehyde. The diamines are condensed with phosgene to give methylene diphenyl diisocyanate, a precursor to urethane polymers.
Other uses include rubber processing chemicals (9%), herbicides (2%), and dyes and pigments (2%). As additives to rubber, aniline derivatives such as phenylenediamines and diphenylamine, are anti…
History
Aniline was first isolated in 1826 by Otto Unverdorben by destructive distillation of indigo. He called it Crystallin. In 1834, Friedlieb Runge isolated a substance from coal tar that turned a beautiful blue color when treated with chloride of lime. He named it kyanol or cyanol. In 1840, Carl Julius Fritzsche (1808–1871) treated indigo with caustic potash and obtained an oil that he named aniline, after an indigo-yielding plant, anil (Indigofera suffruticosa). In 1842, Nikolay Nikolaevich …
Toxicology and testing
Aniline is toxic by inhalation of the vapour, ingestion, or percutaneous absorption. The IARC lists it in Group 3 (not classifiable as to its carcinogenicity to humans) due to the limited and contradictory data available. The early manufacture of aniline resulted in increased incidents of bladder cancer, but these effects are now attributed to naphthylamines, not anilines.
Aniline has been implicated as one possible cause of forest dieback.
Notes
1. ^ Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 416, 668. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4. Aniline, for C6H5-NH2, is the only name for a primary amine retained as a preferred IUPAC name for which full substitution is permitted on the ring and the nitrogen atom. It is a Type 2a retained name; for the rules of substitution see P-…