
A cofactor is a non-protein chemical that assists with a biological chemical reaction. Co-factors may be metal ions, organic compounds, or other chemicals that have helpful properties not usually found in amino acids. Some cofactors can be made inside the body, such as ATP
Adenosine triphosphate
Adenosine triphosphate is a complex organic chemical that provides energy to drive many processes in living cells, e.g. muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all forms of life, ATP is often referred to as the "molecular unit of currency" of i…
What can be cofactors?
cofactor. a co-factor is a non-protein “helper molecule ” that a protein needs in order to be functional. Cofactors can be inorganic (non-hydrocarbon-based) such as metal ions or organic (carbohydron-based). When the cofactor is a metal, we call the metal-protein combo a metalloprotein – or a metalloenzyme if the protein is an enzyme.
Can a protein be a cofactor?
Therefore, vitronectin is the first protein described to function as a cofactor for serpin specificity. PAI-1 is proposed to be a versatile inhibitor which, in the presence of vitronectin, can modulate both coagulation and fibrinolysis. Amino Acid Sequence
What is a growth factor in biology?
Growth factors, which generally considered as a subset of cytokines, refer to the diffusible signaling proteins that stimulate cell growth, differentiation, survival, inflammation, and tissue repair. They can be secreted by neighboring cells, distant tissues and glands, or even tumor cells themselves.
Is a cofactor an inorganic ion?
There are two types of cofactors: inorganic ions [e.g., zinc or Cu (I) ions] and organic molecules known as coenzymes. Most coenzymes are vitamins or are derived from vitamins. Vitamins are organic compounds that are essential in very small (trace) amounts for the maintenance of normal metabolism.
What is a cofactor in biology examples?
Vitamins, minerals, and ATP are all examples of cofactors. ATP functions as a cofactor by transferring energy to chemical reactions.
What are cofactors with example?
Co-factor. A non-protein chemical compound that is required for the activity of an enzyme e.g., zinc ion, copper ion, potassium ion etc.
What is function of cofactor?
Cofactors can be metals or small organic molecules, and their primary function is to assist in enzyme activity. They are able to assist in performing certain, necessary, reactions the enzyme cannot perform alone. They are divided into coenzymes and prosthetic groups.
What do you mean by cofactor in enzymes?
Definition and Examples of Enzyme Cofactor. A cofactor is a non-protein molecule that supports a biochemical reaction. Cofactors can take the form of metal ions, organic substances or other molecules with beneficial characteristics not typically present in amino acids.
What are the 3 types of cofactors?
Co-factors are of three kinds. They are
i) Prosthetic groups ii) Co-enzymes iii) Metal ions .
i) Prosthetic groups : Prosthetic groups are the organic co factors which are tightly bound to the apoenzyme.
What's the difference between cofactor and enzyme?
Some enzymes require the addition of another non-protein molecule to function as an enzyme. These are known as cofactors, and without these enzymes remain within the inactive “apoenzyme” forms. Once the cofactor is added, the enzyme becomes the active “holoenzyme”.
What is difference between cofactor and coenzyme?
Cofactors are non-protein chemical compounds which are termed helper molecules. They are used as a catalyst in reaction and are extremely important. There are two types of cofactors viz coenzymes and prosthetic groups. Coenzymes are defined as organic molecules, small, non-protein which are also termed cosubstrates.
What do cofactors and coenzymes do?
Coenzymes and cofactors are molecules that help an enzyme or protein to function appropriately. Coenzymes are organic molecules and quite often bind loosely to the active site of an enzyme and aid in substrate recruitment, whereas cofactors do not bind the enzyme.
What is cofactor in simple words?
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations.
What are cofactors and coenzymes give examples?
Cofactors are non-protein chemical compounds which are termed helper molecules. They are used as a catalyst in reaction and are extremely important. There are two types of cofactors viz coenzymes and prosthetic groups. Coenzymes are defined as organic molecules, small, non-protein which are also termed cosubstrates.
What are some examples of cofactors and coenzymes?
Vitamins and derivativesCofactorVitaminChemical group(s) transferredCoenzyme APantothenic acid (B5)Acetyl group and other acyl groupsTetrahydrofolic acidFolic acid (B9)Methyl, formyl, methylene and formimino groupsMenaquinoneVitamin KCarbonyl group and electronsAscorbic acidVitamin CElectrons9 more rows
What is cofactor in biology class 11?
Complete answer: Cofactors are the non-protein constituents bound to the enzyme to make the enzyme catalytically active and the protein part of the enzyme is known as apoenzyme. A complete conjugate enzyme, consisting of an apoenzyme and a cofactor is called a holoenzyme.
What is a coenzyme and give one example?
A coenzyme requires the presence of an enzyme in order to function. It is not active on its own. While enzymes are proteins, coenzymes are small, nonprotein molecules. Coenzymes hold an atom or group of atoms, allowing an enzyme to work. Examples of coenzymes include the B vitamins and S-adenosyl methionine.
What is a cofactor in biology?
A cofactor is any molecule that when complexed with another biological molecule and is necessary for the proper functioning of the protein/enzyme.
What are some examples of cofactors and coenzymes?
Enzyme cofactors can be inorganic metal ions such as copper and zinc, compounds such as sulfur-iron complexes, or organic molecules such as vitamins.
What are the types of enzyme cofactors?
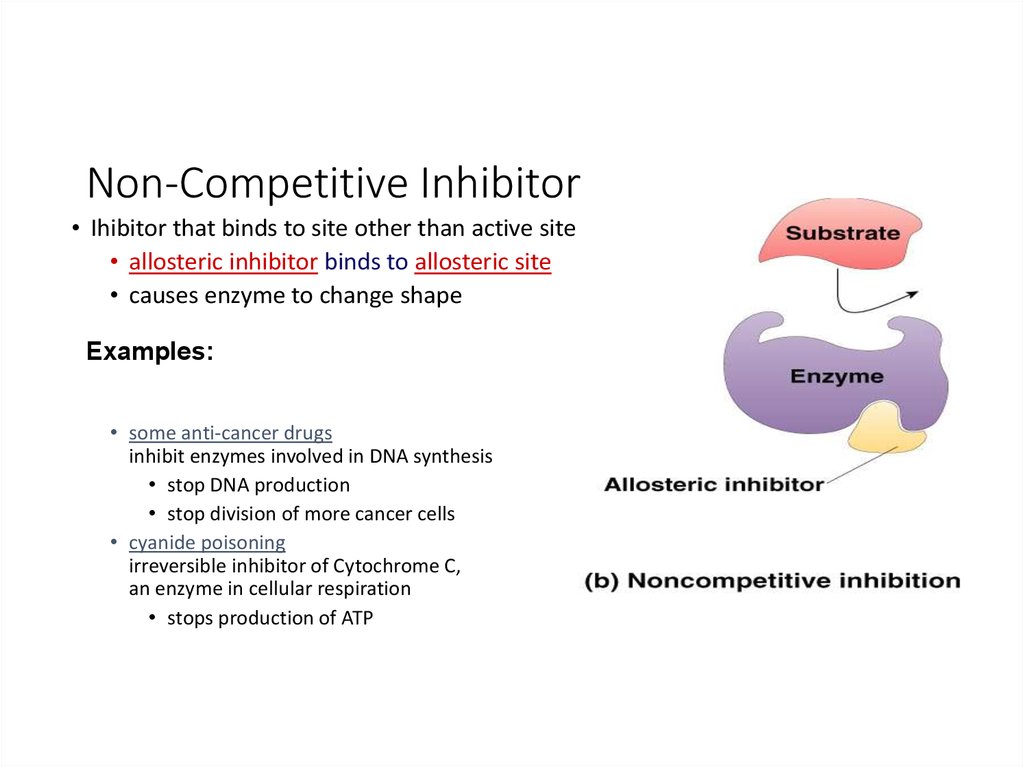
Cofactors can be classified into cofactors, coenzymes, and prosthetic groups. In general, cofactors bind allosterically, coenzymes bind to the acti...
Is every cofactor a coenzyme?
No. Cofactors can bind allosterically to modify the structure of the active site, regulating the enzyme's activity, and are not required for enzyme...
What is a cofactor in biochemistry?
Here, cofactors were defined as an additional substance apart from protein and substrate that is required for enzyme activity and a prosthetic group as a substance that undergoes its whole catalytic cycle attached to a single enzyme molecule. However, the author could not arrive at a single all-encompassing definition of a "coenzyme" and proposed that this term be dropped from use in the literature.
What are cofactors made of?
Cofactors can be divided into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Note that some scientists limit the use of the term "cofactor" to inorganic substances; both types are included here. )
What is the difference between a coenzyme and a prosthetic group?
On the other hand, "prosthetic group" emphasizes the nature of the binding of a cofactor to a protein (tight or covalent) and, thus , refers to a structural property. Different sources give slightly different definitions of coenzymes, cofactors, ...
What are the two types of coenzymes?
The second type of coenzymes are called "cosubstrates", and are transiently bound to the protein. Cosubstrates may be released from a protein at some point, ...
What was the first organic cofactor?
The first organic cofactor to be discovered was NAD +, which was identified by Arthur Harden and William Young 1906. They noticed that adding boiled and filtered yeast extract greatly accelerated alcoholic fermentation in unboiled yeast extracts. They called the unidentified factor responsible for this effect a coferment. Through a long and difficult purification from yeast extracts, this heat-stable factor was identified as a nucleotide sugar phosphate by Hans von Euler-Chelpin. Other cofactors were identified throughout the early 20th century, with ATP being isolated in 1929 by Karl Lohmann, and coenzyme A being discovered in 1945 by Fritz Albert Lipmann.
What is a cofactor in group transfer?
Each class of group-transfer reaction is carried out by a particular cofactor, which is the substrate for a set of enzymes that produce it, and a set of enzymes that consume it. An example of this are the dehydrogenases that use nicotinamide adenine dinucleotide (NAD +) as a cofactor. Here, hundreds of separate types of enzymes remove electrons from their substrates and reduce NAD + to NADH. This reduced cofactor is then a substrate for any of the reductases in the cell that require electrons to reduce their substrates.
What is a cofactor in enzymes?
The succinate dehydrogenase complex showing several cofactors, including flavin, iron-sulfur centers, and heme. A cofactor is a non- protein chemical compound or metallic ion that is required for an enzyme 's activity as a catalyst (a catalyst is a substance that increases the rate ...
Which cofactor inhibits thrombin?
heparin cofactor IIa member of the serpingroup that inhibits thrombin.
What is the definition of coenzyme?
1. One of two or more contributing factors. 2. A substance, such as a metallic ion or coenzyme, that must be associated with an enzyme for the enzyme to function . The American Heritage® Medical Dictionary Copyright © 2007, 2004 by Houghton Mifflin Company. Published by Houghton Mifflin Company. All rights reserved.
What is the point of the analysis of the Jacobian matrix?
The point of the analysis is that the cofactorsof the Jacobian matrix can have their own, sometimes decisive, nonparametric properties.
What does "associate" mean?
1. One of two or more contributing factors.
Is circumcision a cofactor?
The findings suggest that circumcision is an important cofactorin human papillomavirus (HPV) infection and cervical cancer, reported Dr.
Is A5 519W a cofactor?
From this observation we conclude that apo A5 519W is a crucial cofactorfor developing hypertriglyceridemia in patients with apo E2/2.
Is zinc a cofactorin?
The premise is that since zinc is a cofactorin cellular proliferation, including it in dressings should be beneficial.
What Is a Cofactor?
Cofactors are inorganic or small organic molecules that bind enzymes to enable or enhance their activity. Common inorganic cofactors are metals, including but not limited to magnesium, manganese, zinc, molybdenum, cobalt, and copper.
Coenzymes
Coenzymes are organic molecules that bind to the active site of an enzyme, not only facilitating the binding of the enzyme's product but also participating in the enzymatic reaction by either donating or accepting an element or compound during the reaction.
Which Helper Am I?
As you have learned, many enzymes require a non-protein helper to catalyze a biochemical reaction. These helpers are categorized as cofactors, coenzymes, and prosthetic groups. It can be difficult to distinguish between these helpers, as coenzymes and prosthetic groups are cofactor sub-types.
What is the most important coenzyme in the cell?
One of the most important coenzymes in the cell is the hydrogen acceptor nicotinamide adenine dinucleotide (NAD). It is made from vitamin B. NAD acquires a hydrogen atom from an enzyme and it reduces to NADH.
Which molecule contains energy?
The electron of the hydrogen atom contains energy. This energy is carried by NADI- molecule. ,For example, various foods are oxidized in the cell. The cell remo‘ es electrons from the food molecules. It transfers this electron to NAD. It reduces to NADH.
What is a cofactor?
Cofactor is a non-protein chemical compound that tightly and loosely binds with an enzyme or other protein molecules. Basically, cofactors are split into two groups: coenzymes and prosthetic groups (ions usually). Comment on Andrei's post “Both are molecules/ions t...”. Button opens signup modal.
What are the cofactors and coenzymes?
The cofactors and coenzymes (organic cofactors) that help enzymes catalyze reactions.
What are cofactors in biology?
Cofactors are non-protein chemical compounds which are termed as helper molecules. They are used as a catalyst in reaction and are extremely important. There are two types of cofactors viz coenzymes and prosthetic groups. Coenzymes are defined as organic molecules, small, non-protein which are also termed as cosubstrates.
What is the difference between cofactor and coenzyme?
Difference Between Cofactor And Coenzyme. The human body is composed of billions of cells, enzymes, units, etc. Enzymes are the proteins which are necessary to control metabolic as well as chemical reactions of a body. Apart from enzymes, some other compounds involved in such reactions are cofactors and coenzymes.
What are some examples of coenzymes?
They act as carriers and can be easily removed from. Some examples of coenzymes are vitamin-b, coenzyme A, biotin, etc. Also Refer: E nzymes.
What are cofactors in biochemistry?
Some metallic elements have no nutritional value, but several trace elements function as cofactors in biochemical reactions, including iron, copper, zinc, magnesium, cobalt, and molybdenum.
Which trace elements do not act as cofactors?
Some trace elements that appear to be important for nutrition do not appear to act as cofactors, including chromium, iodine, and calcium . Cosubstrates are coenzymes that bind tightly to a protein, yet will be released and bind again at some point.
What is the term used to describe an enzyme that is complete with its coenzymes and cofactors?
There are a few related terms also related to coenzymes: Apoenzyme is the name given to an inactive enzyme that lacks its coenzymes or cofactors. Holoenzyme is the term used to describe an enzyme that is complete with its coenzymes and cofactors. Holoprotein is the word used for a protein with a prosthetic group or cofactor.
What is the difference between coenzymes and enzymes?
They are intermediate carriers of an atom or group of atoms, allowing a reaction to occur. Coenzymes are not considered part of an enzyme's structure. They are sometimes referred to as cosubstrates . Coenzymes cannot function on their own and require the presence of an enzym e.
What is the word used for a protein with a prosthetic group or cofactor?
Holoprotein is the word used for a protein with a prosthetic group or cofactor.
What are some examples of nonvitamin coenzymes?
An example of a nonvitamin coenzyme is S-adenosyl methionine, which transfers a methyl group in bacteria as well as in eukaryotes and archaea.
Is coenzyme a protein?
It is not active on its own. While enzymes are proteins, coenzymes are small, nonprotein molecules. Coenzymes hold an atom or group of atoms, allowing an enzyme to work. Examples of coenzymes include the B vitamins and S-adenosyl methionine.

Introduction
Table of Contents
Definition and Examples of Enzyme Cofactor
- A cofactor is a non-protein molecule that supports a biochemical reaction. Cofactors can take the form of metal ions, organic substances or other molecules with beneficial characteristics not typically present in amino acids. While some cofactors, like ATP, can be produced by the body, others must be obtained through food. Cofactors play an importa...
Types of Enzyme Cofactors
- Vitamins
Vitamins are organic substances that act as cofactors in essential metabolic processes. Since the body cannot produce vitamins, they must usually be obtained through diet. Numerous vitamins serve as cofactors to enable enzymes to catalyse processes, including the production of essenti… - Minerals
Like vitamins, minerals are substances that must be consumed for our cells to function effectively. The difference is that minerals are inorganic compounds that naturally occur and are commonly found in rocks and soil, whereas vitamins are organic molecules, containing carbon, …
Functions of Enzyme Cofactors
- Cofactors usually have the function of contributing chemical groups or qualities that are absent from other chemical groups. For example, the cofactor ATP can transfer energy, which it uses to conduct chemical reactions like enzyme activities and protein transportation. On the other hand, heme is an iron-containing chemical complex which connects with oxygen molecules. Our bloo…
Coenzyme Definition and Examples
- A coenzyme is a chemical that works with the enzyme to start or support the enzyme’s activity. It can be considered as a supporting molecule in a biochemical process. Coenzymes are tiny, non-proteinaceous substances that serve as a transfer site for an active enzyme. They operate as intermediary carriers for an atom or group of atoms, enabling a reaction to take place. Coenzym…
Overview
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically di…
Non-enzymatic cofactors
The term is used in other areas of biology to refer more broadly to non-protein (or even protein) molecules that either activate, inhibit, or are required for the protein to function. For example, ligands such as hormones that bind to and activate receptor proteins are termed cofactors or coactivators, whereas molecules that inhibit receptor proteins are termed corepressors. One such example is the G protein-coupled receptor family of receptors, which are frequently found in sen…
Classification
Cofactors can be divided into two major groups: organic cofactors, such as flavin or heme; and inorganic cofactors, such as the metal ions Mg , Cu , Mn and iron–sulfur clusters.
Organic cofactors are sometimes further divided into coenzymes and prosthetic groups. The term coenzyme refers specifically to enzymes and, as such, to the functional properties of a protein. On the other hand, "prosthetic group" emphasizes the nature of the binding of a cofactor to a protei…
Inorganic cofactors
Metal ions are common cofactors. The study of these cofactors falls under the area of bioinorganic chemistry. In nutrition, the list of essential trace elements reflects their role as cofactors. In humans this list commonly includes iron, magnesium, manganese, cobalt, copper, zinc, and molybdenum. Although chromium deficiency causes impaired glucose tolerance, no human enzyme t…
Organic
Organic cofactors are small organic molecules (typically a molecular mass less than 1000 Da) that can be either loosely or tightly bound to the enzyme and directly participate in the reaction. In the latter case, when it is difficult to remove without denaturing the enzyme, it can be called a prosthetic group. It is important to emphasize that there is no sharp division between loosely and tig…
Protein-derived cofactors
In a number of enzymes, the moiety that acts as a cofactor is formed by post-translational modification of a part of the protein sequence. This often replaces the need for an external binding factor, such as a metal ion, for protein function. Potential modifications could be oxidation of aromatic residues, binding between residues, cleavage or ring-forming. These alterations are distinct from other post-translation protein modifications, such as phosphorylation, …
See also
• Enzyme catalysis
• Inorganic chemistry
• Organometallic chemistry
• Bioorganometallic chemistry
• Cofactor engineering
Further reading
• Bugg T (1997). An introduction to enzyme and coenzyme chemistry. Oxford: Blackwell Science. ISBN 978-0-86542-793-8.