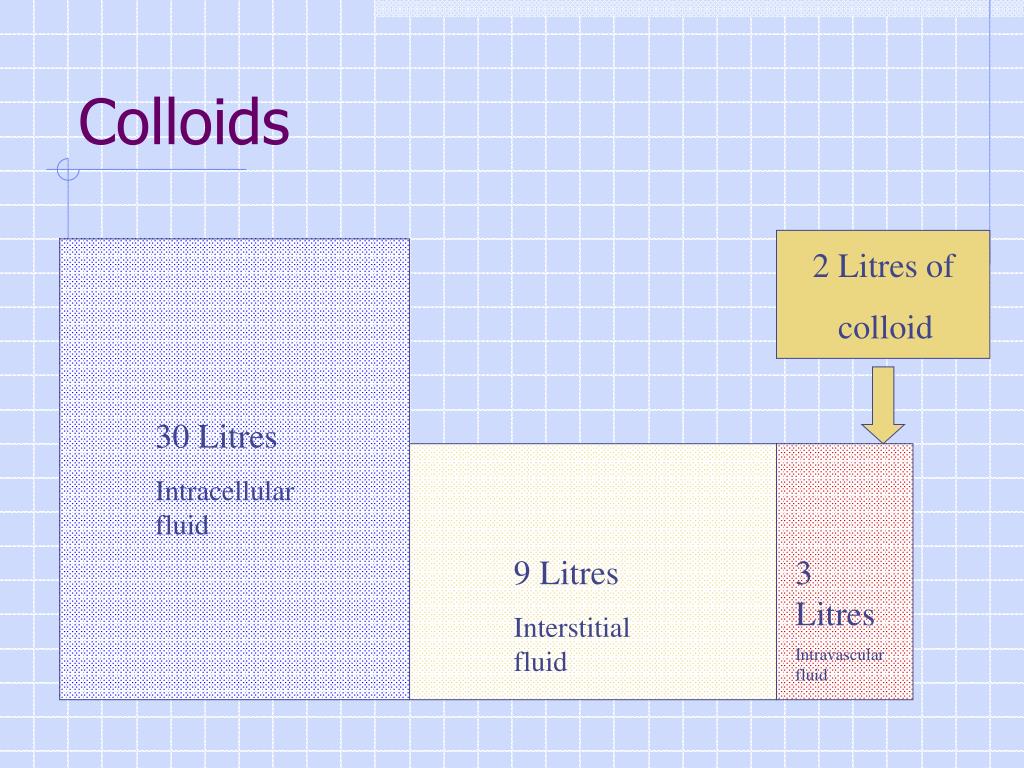
Why are colloids used?
Colloids are often used to replace and maintain intravascular colloid osmotic pressure (COP) and decrease edema that can result from the use of crystalloid fluids. Colloids are rarely used alone, however; they are typically used in conjunction with crystalloid fluids.
Why are colloids used in medicine?
Albumin, dextran, gelatin, and hydroxyethyl starch (HES) solutions are colloids that efficiently expand the circulating blood volume. The administration of colloids restores the intravascular volume with minimal risk of tissue edema in comparison with crystalloid solutions alone.
Why are colloid fluids used to treat shock?
Colloid containing solutions seem superior to crystalloids due to efficient reexpansion of circulating blood volume and enhancement of capillary blood flow. Resuscitation times and thereby the cellular hypoxic insult are considerable reduced while at the same time the formation of excessive tissue oedema is prevented.
What are types of colloid fluids?
Colloids are of two types: Natural, i.e., human albumin. Artificial, i.e., gelatin and dextran solutions, hydroxyethyl starches (HES).
What are 5 uses of colloids?
What are the Applications of Colloids?1) Medicines: Medicines in colloidal form are easily adsorbed by the body tissues and hence are more effective.2) Sewage disposal: ... 3) Purification of water: ... 4) Cleansing action of soap: ... 5) Formation of Delta: ... 6) Smoke precipitation: ... 7) Photography: ... 8 ) Artificial rain:More items...
What does colloid mean in medical terms?
1 : a gelatinous or mucinous substance found in tissues in disease or normally (as in the thyroid) 2a : a substance consisting of particles that are dispersed throughout another substance and are too small for resolution with an ordinary light microscope but are incapable of passing through a semipermeable membrane.
What is the difference between crystalloid and colloid fluids?
Colloids are those substances which are not easily crystallized from their aqueous solutions. Crystalloids are those substances which are easily crystallized from their aqueous solution. Colloids contain much larger particles than crystalloids (1 – 200 nm).
What IV fluid is best for sepsis?
Answer: Crystalloid solutions remain the resuscitative fluid of choice for patients with sepsis and septic shock. Balanced crystalloid solutions may improve patient-centered outcomes and should be considered as an alternative to 0.9% normal saline (when available) in patients with sepsis.
What is the best IV fluid to give for shock?
Both 0.9% saline and Ringer's lactate are equally effective; Ringer's lactate may be preferred in hemorrhagic shock because it somewhat minimizes acidosis and will not cause hyperchloremia. For patients with acute brain injury, 0.9% saline is preferred.
What IV fluids are colloids?
Different types of colloids may be grouped as synthetic or semi‐synthetic, for example: starches, dextrans, gelatins; or naturally occurring, such as human albumin or fresh frozen plasma (FFP).
What are the 4 types of colloid?
The types of colloids includes sol, emulsion, foam, and aerosol.Sol is a colloidal suspension with solid particles in a liquid.Emulsion is between two liquids.Foam is formed when many gas particles are trapped in a liquid or solid.Aerosol contains small particles of liquid or solid dispersed in a gas.
What are 3 examples of a colloid?
Examples of ColloidsColloids refer to dispersions of small particles usually with linear dimensions from around 1 nm to 10 micrometres. ... Examples: fog, smog, and sprays.Examples: smoke and dust in the air.Examples: milk and mayonnaise.Examples: pigmented plastics.Examples: silver iodide sol, toothpaste, and Au sol.More items...
What are colloids in pharmacology?
Colloids are high-molecular-weight substances contained in a high-sodium solution, usually 0.9% saline. Unlike crystalloids, colloids will not readily diffuse through the vascular endothelium and will thus stay in the intravascular space longer than crystalloids.
What are colloids in pharmacy?
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels.
Why are some studies at risk of bias?
Some studies were at risk of bias because some participants in the crystalloid group were given, or may have been given additional colloids. We excluded two studies from analysis of the primary outcome ( Annane 2013; Ngo 2001 ). This did not alter interpretation of the effect for all‐cause mortality (at the end of follow‐up), with little or no difference between groups (RR 0.94, 95% CI 0.52 to 1.72; 143 participants; 4 studies; I² = 0%).
Why do people lose fluid?
Critically ill people may lose fluid because of serious conditions, infections (e.g. sepsis), trauma, or burns, and need additional fluids urgently to prevent dehydration or kidney failure. Colloid or crystalloid solutions may be used for this purpose.
What is a colloidal fluid?
Colloids and crystalloids are types of fluids that are used for fluid replacement, often intravenously (via a tube straight into the blood). Crystalloids are low‐cost salt solutions (e.g. saline) with small molecules, which can move around easily when injected into the body.
Why do people need colloids?
Critically ill people may lose large amounts of blood (because of trauma or burns), or have serious conditions or infections (e.g. sepsis); they require additional fluids urgently to prevent dehydration or kidney failure.
How much NaCl is in Dextran 70?
Details: 7.5 % NaCl in 6% dextran 70; 250 mL bags; fluid infused at a wide‐open rate; conventional fluids also given if necessary
What is the condition where you lose fluids?
Description of the condition. Critically ill people may experience excessive fluid loss, and hypovolaemia, because of haemorrhage from serious injury or burns , or because of critical illnesses, which lead to dehydration, vomiting, or diarrhoea.
How many mL of fluid is given in 90 min?
Additional details: fluids given during 90 min on basis of response to predefined pressure limits and CVP, according to a protocol; boluses at maximum of 200 mL/10 min, so that maximum fluid challenge was 1800 mL in 90 min
What are the components of the Starling-Landis equation that affect intravascular water content?
Figure 2-1 The main components of the Starling-Landis equation that affect intravascular water content include intravascular hydrostatic pressure (HP) and colloid osmotic pressure (COP). The intravascular HP is a result of intravascular volume, cardiac output (CO), and systemic vascular resistance (SVR). Under normal conditions the HP favors the movement of fluid from the vessel into the interstitium. The COP is the force that opposes intravascular HP, supporting intravascular water retention. The COP is generated by the presence of large molecules (primarily proteins) that do not readily pass the capillary membrane and create an osmotic effect.
What are the components of intravascular fluid volume?
The main components that control intravascular fluid volume include intravascular colloid osmotic pressure (COP) and hydrostatic pressure (HP) (Figure 2-1). Eighty percent of the COP is produced by albumin, which is the most abundant extracellular protein. The pressure generated by albumin is augmented by its negative charge, which attracts cations (e.g., sodium) and water around its core structure. This unique dynamic is termed the Gibbs – Donnan effect. Vascular permeability to ion species, ionic concentration gradients, and electrochemical charges influence the movement of ions such as sodium, potassium, and chloride. Capillary membrane pore size and the filtration coefficient control the ease with which larger molecules such as albumin leave the intravascular space. Pore size varies from tissue to tissue (e.g., continuous capillaries in the brain and fenestrated capillaries in the liver). The filtration coefficient is variable and partly dependent on the amount of albumin in the intravascular space and within the interendothelial cleft.
What is the goal of resuscitation and maintenance fluid therapy?
The goals of resuscitation and maintenance fluid therapy in the critically ill animal are to restore and maintain perfusion and hydration without causing volume overload and complications caused by pulmonary, peripheral, or brain edema. By using colloid fluids in conjunction with crystalloid fluids (see Chapter 1), goal-driven resuscitation (also known as end-point resuscitation) can be achieved more rapidly and with less fluid volume compared with crystalloid fluids alone. Maintaining an effective circulating volume can be challenging when there is vascular leakage, vasodilation, excessive vasoconstriction, inadequate cardiac function, hypoalbuminemia, or ongoing fluid loss. Whether a fluid administered intravenously remains in the intravascular compartment or moves into the interstitial or intracellular spaces depends on the dynamic forces that affect fluid movement between body fluid compartments.
Why is 25% albumin important?
Because of its high concentration of albumin and high COP (200 mm Hg), 25% human albumin has the greatest capability for increasing plasma COP. When capillary permeability is normal, 25% albumin can be an effective colloid for rapid intravascular volume expansion. It also can be used to minimize interstitial edema in animals with hypoalbuminemia caused by inadequate albumin production or renal and gastrointestinal albumin loss. However, when increased capillary permeability allows plasma albumin to pass into the interstitium, the initial intravascular COP benefits of albumin infusion are temporary and increased interstitial COP and edema may result.
What is the result of intravascular volume depletion?
Severe intravascular volume depletion associated with conditions such as hemorrhage, trauma, systemic inflammatory response syndrome (SIRS) diseases, and various metabolic diseases ultima tely results in poor tissue perfusion, tissue hypoxia, and cellular energy depletion. As a consequence, vascular tone can be lost and capillary permeability can be increased, leading to maldistribution of fluid between fluid compartments. Timely and appropriate intravascular fluid resuscitation becomes the mainstay of treatment to restore perfusion and oxygen delivery.
What is the force that opposes intravascular HP, supporting intravascular water retention?
The COP is the force that opposes intravascular HP, supporting intravascular water retention. The COP is generated by the presence of large molecules (primarily proteins) that do not readily pass the capillary membrane and create an osmotic effect.
What influences the movement of ions?
Vascular permeability to ion species, ionic concentration gradients, and electrochemical charges influence the movement of ions such as sodium, potassium, and chloride. Capillary membrane pore size and the filtration coefficient control the ease with which larger molecules such as albumin leave the intravascular space.
What are colloids used for?
Colloids are the type on intravenous fluids with high osmolarity that are ideal to transfuse in conditions like decreased intravascular volume. A Few Colloids Examples are: 1 Dextrans (Lomodex) 2 Albumins 3 Gelatins (Haemaccel) 4 Hydroxyethyl Starch 5 Perflurocarbon Emulsions 6 Blood 7 Hextend
What is the Molecular Weight of Haemaccel?
Gelatins (Haemaccel) Colloids Examples that is most commonly used are the gelatins. They have a Molecular weight of 30,000. They are available as 3.5% solution. Composition of Haemaccel: Each liter contains: Gelatin : 35g.
What is the best medication for protein loss?
1. Dextrans (Lomodex) For more on Dextrans read. 2. Albumins. Available as 5% and 25% solution. These are very expensive. Albumin has an intravascular half life of 10-15 days. Used when there is protein loss from body like in:
Does pentastarch interfere with clotting?
6. Blood. Although at clinically used doses they does not interfere with clotting but at high doses (>20m1/kg) they also interfere with clotting. Allergic reactions are less common Allergic reactions and interference with clotting are almost same Pentastarch with as compared with Hexastarch.
How long does a 6% solution last?
Available as 6% and 10% solution. They have prolonged half life and expand plasma effectively for 4 hours.
How long does it take for plasma to expand?
Expand plasma effectively for 2 hours (25% may be present in blood after 12 hours).
Is hextend a colloids?
This is another among the new Colloids Examples. Hextend is another hydroxyethyl starch which also has glucose & lactate but it is under trials and is considered to effect coagulation less than hydroethyl starch.
What does "cool hands" mean?
Hands and feet that are cool to the touch or blotchy looking.
Why do we need water?
When you don’t have enough water in your body, that’s called dehydration. A person needs IV fluids when they become dangerously dehydrated.
What is an IV used for?
The IV also may be used to deliver medications or nutrition.
What are some examples of large molecules that can't easily pass through cell membranes?
Examples include albumin and hetastarch.
How to keep IV needle in place?
Tape the IV needle to your arm so that it stays in place.
What is the most common type of IV fluid?
Crystalloid solutions : These are the most common types of IV fluid. They contain small dissolved molecules that pass easily from the bloodstream into tissues and cells. Examples include normal saline, which is salt in water, and D5W, which is dextrose (sugar) in water. Another example is lactated Ringer’s, which contains sodium, chloride, potassium, calcium and lactate. It’s used for aggressive fluid replacement.
How to make blood fill veins?
Tie an elastic band (tourniquet) around your arm to make blood fill the veins.
What is crystalloid fluid?
Crystalloid fluids are a subset of intravenous solutions that are frequently used in the clinical setting. Crystalloid fluids are the first choice for fluid resuscitation in the presence of hypovolemia, hemorrhage, sepsis, and dehydration.
How are crystalloid fluids administered?
Crystalloid fluids are administered parenterally via an intravenous infusion. Infusion rates depend on the clinical presentation and indication for administration. Fluid Resuscitation. In an acute setting, the clinical situation may indicate a rapid infusion of crystalloid fluids.
What is EGDT in septic shock?
In 2012, the Surviving Sepsis Campaign guidelines recommended Early Goal-directed Therapy (EGDT) as the standard of care in managing patients in septic shock. These guidelines dictated that patients receive empirically dosed rapid volume resuscitation. Patients should receive a fluid challenge of 20 mL/kg over the first 30 minutes of treatment. Subsequent volume dosing should depend on the severity of hypovolemia and should be adjusted in increments of 500 mL, aiming for an ultimate central venous pressure of 8 to 12 mmHg.[1] However, revised guidelines in 2018 have called into question the efficacy of EGDT. The revised guidelines now state that fluid provision should be in a 1-hour bundle by administering 30 mL/kg crystalloid for hypotension or lactate 4 mmol/L. This guideline has a rating of 'strong recommendation.'
What is intravenous fluid?
Broadly, intravenous fluids can fall into two separate categories: crystalloids and colloids. In most clinical settings, crystalloids are the choice of fluid for many indications for fluid resuscitation, maintenance, or as a solvent for medication delivery.
What are the three molecules in buffered solutions?
These solutions were designed to sustain a normal physiologic plasma pH. The three commonly used molecules are lactate, acetate, and gluconate. Lactate and gluconate are hepatically metabolized to bicarbonate, while acetate is predominantly metabolized peripherally by skeletal muscle.
Why are buffered solutions osmotically active?
Because of this discrepancy in concentration, these fluids are osmotically active and will cause fluid shifts. Their primary indication is for emergent replacement of serum solutes, such as in hyponatremia with neurologic symptoms. Buffered solutions contain molecules that metabolize in vivo to bicarbonate.
When was the mass based formula for maintenance crystalloid fluids developed?
The fluid requirements of patients were determined to be related to a patient's caloric demand by Drs. Holliday and Segar in 1957.[2] Since this time, their initial formula has been modified to provide clinicians with guidelines for administering maintenance crystalloid fluids. The mass-based formula uses what is known as the "4-2-1" rule:
Why are colloids used in medicine?
They’re also called volume or plasma expanders, because they draw fluid from the interstitial space back into the blood vessels with oncotic pressure. Because colloids require less volume than crystalloid solutions, they are used for patients who are unable to tolerate large fluid volumes, or are malnourished.
What is the most common solution used in osmosis?
Crystalloid Solutions: Most Commonly Used. Crystalloid solutions contain small particles that that pass easily from the bloodstream to cells and tissues. There are three types of crystalloids, given according to their tonicity, the ability to make water move into or out of a cell by osmosis.
How many crystalloids are administered?
Although crystalloids are administered routinely, which solution is ordered depends on the patient’s condition. Four solutions are the most commonly administered. Here is a brief description of each:
How does water move in cells?
Water will move from extracellular space into the cells. Hypertonic: When the extracellular fluid has more solutes (osmolarity) than within the cells, water flows out of the cells. Isotonic: Both the extracellular and intracellular fluids have the same osmolarity, so there is no movement of water between them.
What happens when fluid is lost?
Joint lubrication. When fluid is lost for any reason, electrolytes become imbalanced, body systems are stressed, and cognitive function in the brain is impaired. Blood becomes concentrated, signaling the kidneys to retain water. As a result, urine output is decreased.
What happens when you lose water?
When water is lost, IV solutions restore fluid balance. The human body is made up of about 60% water, with two-thirds of it stored intracellularly. The rest is found in blood vessels and between the cells. Water makes up 73% of the brain and heart; 83% of the lungs; 79% of the muscles and kidneys; and 64% of the skin.
What is IV therapy?
All nursing programs include fluid balance and intravenous (IV) therapy as part of the curriculum. The information about the types of IV solutions and when to use them can be confusing for a nursing student. Nurse.Plus is happy to offer this simple reference guide to the four basic types.
What are IV Fluids?
Intravenous fluids, also known as intravenous solutions, are supplemental fluids used in intravenous therapy to restore or maintain normal fluid volume and electrolyte balance when the oral route is not possible . IV fluid therapy is an efficient and effective way of supplying fluids directly into the intravascular fluid compartment, in replacing electrolyte losses, and in administering medications and blood products.
What is hypertonic sodium chloride used for?
Hypertonic sodium chloride solutions are used in the acute treatment of sodium deficiency (severe hyponatremia) and should be used only in critical situations to treat hyponatremia. They need to be infused at a very low rate to avoid the risk of overload and pulmonary edema. If administered in large quantities and rapidly, they may cause an extracellular volume excess and precipitate circulatory overload and dehydration. Therefore, they should be administered cautiously and usually only when the serum osmolality has decreased to critically low levels. Some patients may need diuretic therapy to assist in fluid excretion. It is also used in patients with cerebral edema.
What is ringer's solution?
Lactated Ringer’s Solution (also known as Ringer’s Lactate or Hartmann solution) is a crystalloid isotonic IV fluid designed to be the near-physiological solution of balanced electrolytes. It contains 130 mEq/L of sodium, 4 mEq/L of potassium, 3 mEq/L of calcium, and 109 mEq/L of chloride.
How to tell if you have fluid overload?
Observe for signs of fluid overload. Look for signs of hypervolemia such as hypertension, bounding pulse, pulmonary crackles, dyspnea, shortness of breath, peripheral edema, jugular venous distention, and extra heart sounds.
How much mEq/L is 3% NaCl?
3% sodium chloride (3% NaCl) containing 513 mEq/L of sodium and chloride with an osmolality of 1030 mOsm/L.
Why is saline called normal saline?
It is called normal saline solution because the percentage of sodium chloride dissolved in the solution is similar to the usual concentration of sodium and chloride in the intravascular space.
Why are colloids important?
They are useful for expanding the intravascular volume and raising blood pressure. Colloids are indicated for patients in malnourished states and patients who cannot tolerate large infusions of fluid. Colloid IV Fluids and Solutions Cheat Sheet.