
Here are ten examples of homogeneous mixtures:
- Sea water.
- Wine.
- Vinegar.
- Steel.
- Brass.
- Air.
- Natural gas.
- Blood.
Full Answer
What are the three forms of homogeneous mixtures?
ADVERTISEMENT. Homogenous mixtures can be categorized into three different forms: solutions (liquids), gases, and solids . Solutions are homogenous when the ratio of the solvent/solute remains the same, a condition that must hold true even if there are multiple homogenized sources within it.
What is homogeneous mixture?
A homogenous mixture is one that is uniform in composition, one where the mixture’s components have blended together and become distributed uniformly. Examples of homogeneous mixtures include:
How to tell if a mixture is heterogeneous or homogeneous?
To begin with, remember that in the case of a homogenous mixture it doesn’t matter where in the mixture a sample is taken, the composition of the sample will always be the same. By contrast, the sampling location in a heterogeneous mixture will have an impact on the composition of the sample. As an illustrative example, let’s assume you have a large jar full of candies.
What is a solution in chemistry?
Mixtures may be described with terms that reference the particle size of the mixture’s various components. For instance, the classification “solution” is typically given to mixtures that have particles smaller than 1 nm in diameter, with particles that are extremely small in size. Solutions are almost always stable, and the processes of decanting or centrifuging can’t separate the chemical components of the solution form one another. Examples of solutions include gelatin, air, and oxygen dissolved into water.
How is homogenized milk made?
Natural milk will separate into different layers, with the cream on top and the liquid part on the bottom. Natural milk usually only has the appearance of a homogenous mixture when shaken up. To get milk to the condition that it is in when it in on store shelves, it undergoes homogenization. Homogenized milk is processed with special instruments to ensure that the milk’s components don’t separate out and that the milk stays as a homogenous mixture .
What is homogenous suspension?
In chemistry, homogenous suspensions are those that can be divided in half and still possess roughly the same amount of material within both of the halves. While the particles in a homogenous solution cannot be seen with the naked eye, it may be possible to distinguish them with the use of a magnifying lens or microscope.
What happens when two or more substances combine?
Two or more substances combined form a mixture, a compound where the different components have their own chemical identities yet they blend together and create a distribution of the substances. It’s important to know that new bonds aren’t created nor are they broken in a mixture. The chemical properties of a mixture will not change when substances are combined together in the same environment, but the physical properties of the mixture may change.
What is a homogeneous mixture?
For instance, when we want to enjoy the flavors of several fruits altogether, we prepare ourselves a fruit salad. In chemistry, a mixture is formed when two or more chemical substances are combined together in such a way that neither of them loses their chemical identity. Neither the already existing chemical bonds are broken nor new ones are formed. A homogenous mixture is a mixture in which the composition is uniform throughout the mixture. For instance, your fruit salad will be called a homogenous mixture if almost every scoop of it tastes similar. If it doesn’t taste the same, then it will be called a heterogeneous mixture of fruits. Often it is easy to confuse a homogeneous mixture with a pure substance because they both have a uniform composition. The difference is that the composition of a pure substance is always the same, whereas two different homogenous mixtures made from similar molecules can have a different composition. In chemistry, all the solutions are considered homogenous mixtures because the ratio of solute to solvent remains the same throughout the solution, and the particles are not visible with the naked eye, even if homogenized with multiple sources. Let’s take a look at a few homogeneous mixtures that we come across in our daily life.
What is the most common homogeneous mixture in the kitchen?
Vinegar is another most common homogeneous mixture that one can come across in their kitchen. It is an aqueous solution of acetic acid and trace amounts of other chemicals that provide flavor to the vinegar . Typically 5-8% acetic acid is homogeneously mixed with water to make vinegar. Depending on the source material, vinegar in the market comes in several types with distilled white vinegar that looks like clear water being the most common type. Other types of vinegar include apple cider vinegar, white wine vinegar, red wine vinegar, balsamic vinegar , rice vinegar , malt vinegar , etc. Vinegar is most commonly used in cooking to provide a sour taste, acidic flavoring, and for preparing pickles.
Why is it easy to confuse a homogeneous mixture with a pure substance?
Often it is easy to confuse a homogeneous mixture with a pure substance because they both have a uniform composition. The difference is that the composition of a pure substance is always the same, whereas two different homogenous mixtures made from similar molecules can have a different composition.
How is a mixture formed?
In chemistry, a mixture is formed when two or more chemical substances are combined together in such a way that neither of them loses their chemical identity. Neither the already existing chemical bonds are broken nor new ones are formed.
Is black coffee homogeneous?
You don’t want the components to separate, but you want your drink to be uniform from top to bottom. Black coffee is a homogeneous mixture of roasted coffee beans and hot water that can not be physically separated once mixed.
Is fruit salad homogeneous or heterogeneous?
For instance, your fruit salad will be called a homogenous mixture if almost every scoop of it tastes similar. If it doesn’t taste the same, then it will be called a heterogeneous mixture of fruits. Often it is easy to confuse a homogeneous mixture with a pure substance because they both have a uniform composition.
Is Coca Cola a homogeneous mixture?
Any type of soft drink is a homogeneous mixture of solids, liquids, and gas. The first sip of Coca-Cola tastes the same as the last, which implies that the composition of ingredients inside the bottle is uniform throughout; however, in some cases, if the bottle is left open for some time, there can be a variation in taste due to the oxidation of the drink. Nonetheless, a mixture of ice and soft drink is indeed heterogeneous, despite being the same in taste throughout the glass. This is because the ice can be separated from the soft drink at any point, at any point, unless it dissolves itself completely, on which the glass will again be full of a homogene ous mixture.
What are some examples of homogeneous mixtures?
Examples of homogeneous mixtures. Wine: this substance, which contains water, sugar, yeast and fruits that are mixed uniformly is another example of homogeneous mixtures. Preparation of cake : this mixture can be composed of flour, milk, butter, eggs and sugar but, if we observe it with the naked eye we will not be able to identify all these ...
What are the two types of mixtures?
Two types of mixtures can be identified: homogeneous and heterogeneous : 1 Heterogeneous mixtures : These are those in which the substances that make up the mixture (eg oil and water ) can be distinguished at first glance . That is why it is said that they are not uniform. since the substances do not combine. The same goes for a salad of, for example, lettuce and tomato. 2 Homogeneous mixtures : On the other hand, they are characterized by being uniform. That is to say, that the human being will not be able to easily identify that it is at least two substances combined, since there is no discontinuity between them . Eg wine , gelatin , beer , coffee with milk .
Why are homogeneous mixtures not uniform?
The same goes for a salad of, for example, lettuce and tomato. Homogeneous mixtures : On the other hand, they are characterized by being uniform. That is to say, that the human being will not be able to easily identify that it is at least two substances combined, ...
What is the mixture of air and salt?
Air: this mixture is composed of various gaseous substances, such as carbon dioxide, nitrogen, oxygen and ozone, among other gases. Water with salt: in this case, the salt is diluted in water, so it is not possible to detect both substances separately, but they are seen uniformly. Mayonnaise: this dressing contains substances such as egg, ...
Is Invar homogeneous or homogeneous?
Invar: this alloy can also be considered homogeneous since it is composed of nickel and iron. Alnico: it is an alloy composed of cobalt, aluminum and nickel. Advantages and disadvantages of CRM. Unbiased Cost for Being a Wealthy Affiliate.
Is pizza mass homogeneous?
Pizza mass: this dough, which contains flour, yeast, water, salt, among other ingredients, is homogeneous as they are mixed uniformly. Bronze: this alloy is an example of homogeneous substances since it is composed of tin and copper.
Is milk a homogeneous mixture?
Milk : this mixture that we see in a uniform way is composed of substances such as water and fats. Artificial juice : the powdered juices that are prepared with water are one more example of the homogeneous mixtures since they are united in a uniform way.
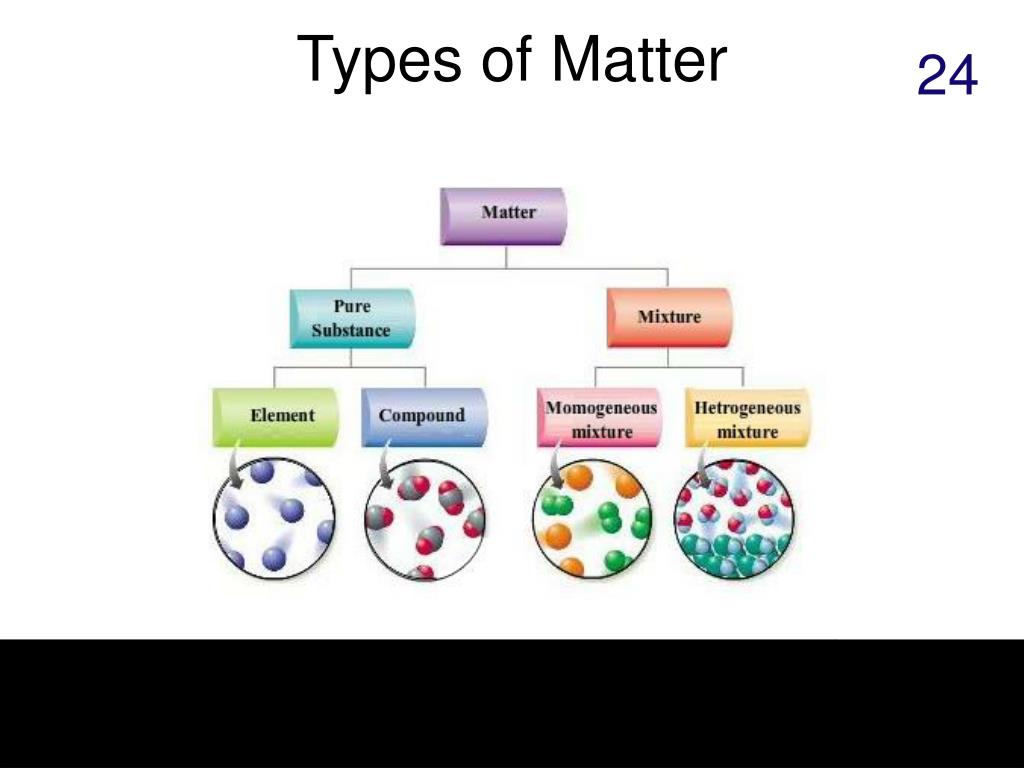
Defining Homogenous Mixtures
Heterogeneous Mixtures
Notes About Mixtures
Terms to Describe Mixtures
The Homogenization Process
Examples of Homogenous Mixtures
- Now let’s look at some specific examples of homogenous mixtures. Homogenous mixtures include mixtures such as laundry detergent, blood plasma, vinegar, and coffee. Blood plasma is a substance found within the blood, and it is what suspends the red blood cells within a fluid. This blood plasma makes up about half of the volume of the blood in the bo...
Examples of Heterogeneous Mixtures
Better Understanding Mixtures