The hydrogen line, 21 centimeter line, or H I line [a] is the electromagnetic radiation spectral line that is created by a change in the energy state of neutral hydrogen atoms.
Full Answer
Why does hydrogen have so many spectral lines?
Though a hydrogen atom has only one electron, it contains a large number of shells, so when this single electron jumps from one shell to another, a photon is emitted, and the energy difference of the shells causes different wavelengths to be released… hence, mono-electronic hydrogen has many spectral lines.
How many spectral lines are present in the hydrogen spectrum?
These are the so-called Balmer series of transitions that take the electron from n = 2 to 3, 4, 5 and 6. The 5th line of this series (n = 2 → 7) occurs at 396.7 nm which is just beyond our ability to see. So, there are four lines in the visible spectrum of hydrogen.
Why are there multiple lines in the hydrogen line spectrum?
When it falls back to the ground state it emits energy radiations with each jump and therefore, spectral lines of different wavelengths (colors) are emitted. This is the reason we see a number of lines in the spectrum of hydrogen.
How are the lines in a hydrogen spectrum obtained?
- α line of Lyman series p = 1 and n = 2
- α line of Lyman series p = 1 and n = 3
- γ line of Lyman series p = 1 and n = 4
- the longest line of Lyman series p = 1 and n = 2
- the shortest line of Lyman series p = 1 and n = ∞

Where are hydrogen spectral lines?
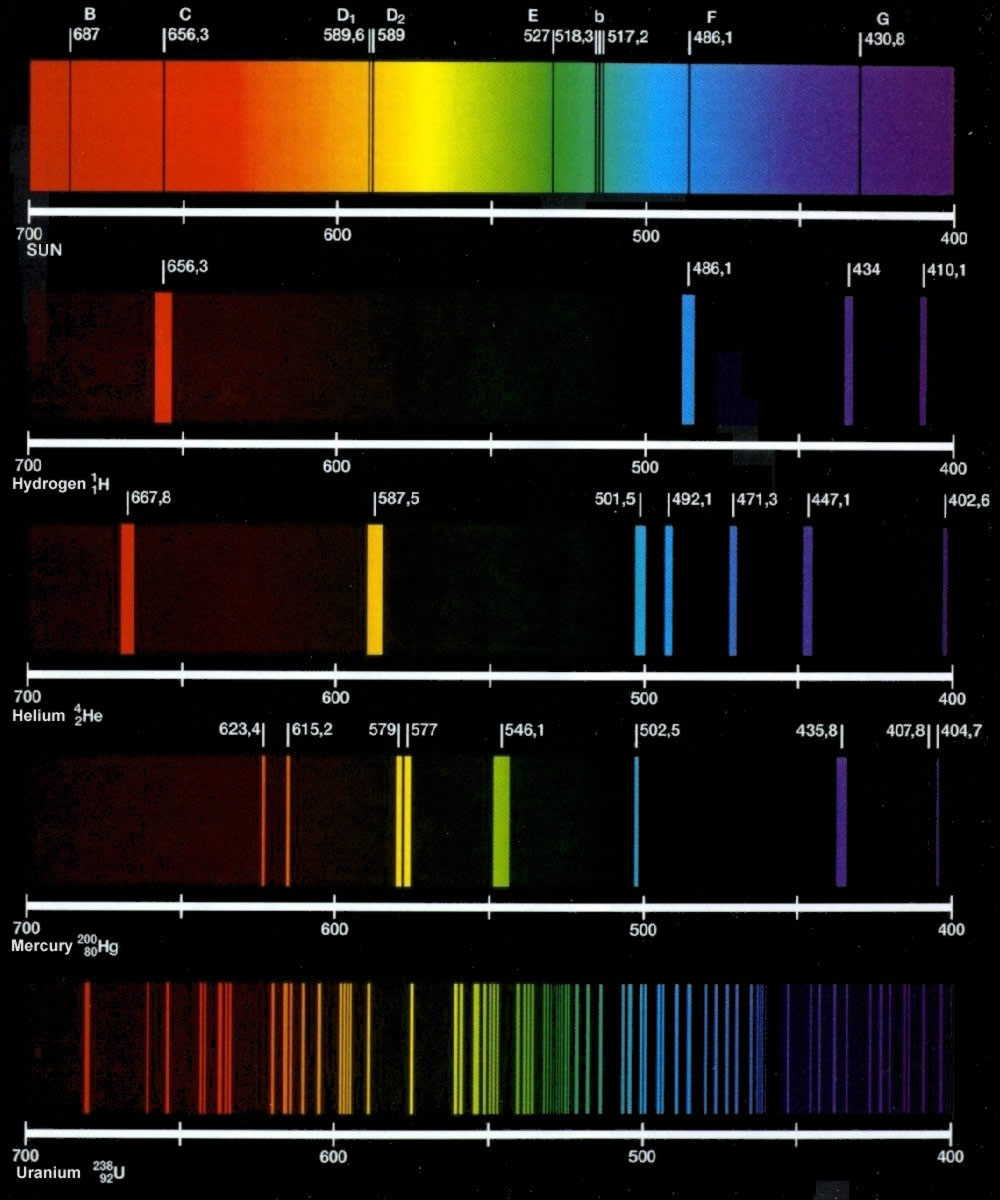
visible spectrumThe light emitted by hydrogen atoms is red because, of its four characteristic lines, the most intense line in its spectrum is in the red portion of the visible spectrum, at 656 nm.
What do you mean by line spectrum of hydrogen?
The emission spectrum of hydrogen or line spectrum of hydrogen is produced when hydrogen gas is taken in the discharge tube and the light emitted on passing electric discharge at low pressure is examined with a spectroscope. It is found to consist of a large number of lines that are grouped into different series.
What is a hydrogen line?
(physics) All the lines in the emission or absorption spectrum of hydrogen; each one corresponding to an allowed transition between quantum energy levels.
What are the spectral lines for hydrogen gas?
Emission Spectrum of HydrogenWavelengthColor656.2red486.1blue-green434.0blue-violet410.1violet
What are spectral lines Class 11?
The wavelengths of various lines show that these lines lie in visible, ultraviolet and infrared regions. All these lines are known as Hydrogen spectral lines. All the spectral lines observed in the hydrogen spectrum can be classified into different series: Lyman, Balmer, Paschen, Brackett and Pfund series.
Why does hydrogen only have 4 spectral lines?
Although hydrogen has only one electron, it contains many energy levels. When its electron jumps from higher energy level to a lower one, it releases a photon. Those photons cause different colours of light of different wavelengths due to the different levels. Those photons appear as lines.
What are spectral lines in chemistry?
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from an excess or deficiency of photons in a narrow frequency range, compared with the nearby frequencies.
How are spectral lines formed?
Spectral lines are produced by transitions of electrons within atoms or ions. As the electrons move closer to or farther from the nucleus of an atom (or of an ion), energy in the form of light (or other radiation) is emitted or absorbed.…
How many spectral lines are there in hydrogen atom?
Hydrogen atom is said to be the simplest atomic system found in nature, thus it produces the simplest of the spectral series. So, the ten lines are; $ 5 \to 4,5 \to 3,5 \to 2,5 \to 1,4 \to 3,4 \to 2,4 \to 1,3 \to 2,3 \to 1,2 \to 1\; $ are possible in this case.
How a hydrogen line spectrum is obtained?
The absorption spectrum is obtained by giving energy to an isolated hydrogen atom and then passing the light energy released by it through a prism to get a spectrum.
What do you mean by Lyman series?
In physics and chemistry, the Lyman series is a hydrogen spectral series of transitions and resulting ultraviolet emission lines of the hydrogen atom as an electron goes from n ≥ 2 to n = 1 (where n is the principal quantum number), the lowest energy level of the electron.
How Bohr explain spectrum of hydrogen?
Niels Bohr explained the line spectrum of the hydrogen atom by assuming that the electron moved in circular orbits and that orbits with only certain radii were allowed. The orbit closest to the nucleus represented the ground state of the atom and was most stable; orbits farther away were higher-energy excited states.
What is the emission spectrum of hydrogen?
The hydrogen emission spectrum comprises radiation of discrete frequencies. These series of radiation are named after the scientists who discovered them.
Why is the hydrogen spectrum important?
The hydrogen spectrum is an important piece of evidence to show the quantized electronic structure of an atom. The hydrogen atoms of the molecule dissociate as soon as an electric discharge is passed through a gaseous hydrogen molecule. It results in the emission of electromagnetic radiation initiated by the energetically excited hydrogen atoms.
What is the name of the series of lines in the electromagnetic spectrum that lies in the visible region?
This series of the hydrogen emission spectrum is known as the Balmer series. This is the only series of lines in the electromagnetic spectrum that lies in the visible region. The value, 109,677 cm -1, is called the Rydberg constant for hydrogen. The Balmer series is basically the part of the hydrogen emission spectrum responsible for ...
How does a hydrogen atom absorb a photon?
When a photon is emitted through a hydrogen atom, the electron undergoes a transition from a higher energy level to a lower , for example, n = 3, n = 2. During this transition from a higher level to a lower level, there is the transmission of light occurs. The quantized energy levels of the atoms, cause the spectrum to comprise wavelengths that reflect the differences in these energy levels. For example, the line at 656 nm corresponds to the transition n = 3 n = 2.
Who derived the wave number of hydrogen spectral line emissions due to the transition of an electron from one orbit to
Similarly, other transitions also have their own series names. Some of them are listed below, Johannes Rydberg , a Swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from one orbit to another.
Who proposed the formula for correlating the wave number of the spectral lines emitted and the energy shells
In the year 1885, on the basis of experimental observations, Balmer proposed the formula for correlating the wave number of the spectral lines emitted and the energy shells involved. This formula is given as:
Spectral Lines of Hydrogen
Bohr’s model explains the spectral lines of the hydrogen atomic emission spectrum. While the electron of the atom remains in the ground state, its energy is unchanged. When the atom absorbs one or more quanta of energy, the electron moves from the ground state orbit to an excited state orbit that is further away.
Summary
Emission lines for hydrogen correspond to energy changes related to electron transitions.
Review
What happens when a hydrogen atom absorbs one or more quanta of energy?
Hydrogen Spectrum: Frequency and Wavelength Relation
The hydrogen emission spectrum can be represented using spectral lines, which is one way to go. Another way is using wavelength to represent the lines. There’s a mathematical relation between the speed of light, frequency, and wavelength. But, this relation forms two different views of the spectrum, i.e., using frequency and wavelength.
Hydrogen Spectrum Wavelength
Whenever a hydrogen atom absorbs a photon, the electron undergoes an energy level transition, for example, n=1, n=2. When a photon passes through a hydrogen atom, the electron transitions from a higher to a lower energy state, for example, n=3, n=2. The transmission of light happens during this transition from a higher to a lower level.
Hydrogen Emission Spectrum Series
The Hydrogen Emission Spectrum is divided into several spectral series, with wavelengths calculated using the Rydberg formula. These spectral lines are formed by electrons in an atom transitioning between two energy levels.
Hydrogen Spectrum Series Formula
A large number of spectral lines are present in the hydrogen spectrum. However, in 1890, Rydberg gave a very simplified theoretical equation for the wavelength of these lines. The equation provides a calculation of the wavenumber v of the lines by the formula:
Things To Remember
When an electron in a hydrogen atom goes from a higher energy level to a lower energy level, the difference in energies between the two levels is emitted as a specific wavelength of radiation. It's known as a spectral line.
Sample Questions
Ques. What is the wavelength of the light emitted when the electron in a hydrogen atom undergoes transition from the energy level with n = 4 to energy level n = 2 ? What is the colour corresponding to this wavelength ? (Given RH = 109678 cm-1) (2 Marks)
Who was the first person to discover the hydrogen line?
In 1959, Italian physicist Giuseppe Cocconi and American physicist Philip Morrison published "Searching for Interstellar Communications", a paper proposing the 21 cm hydrogen line and the potential of microwaves in the search for interstellar communications.
Where is the 21 cm spectral line?
The 21 cm spectral line appears within the radio spectrum ( in the L band of the UHF band of the microwave window to be exact). Electromagnetic energy in this range can easily pass through the Earth's atmosphere and be observed from the Earth with little interference.
What is the hyperfine transition of hydrogen?
The hyperfine transition of hydrogen, as depicted on the Pioneer and Voyager spacecraft. The Pioneer plaque, attached to the Pioneer 10 and Pioneer 11 spacecraft, portrays the hyperfine transition of neutral hydrogen and used the wavelength as a standard scale of measurement.
What frequency does hydrogen produce?
He referred this to Hendrik van de Hulst who, in 1944, predicted that neutral hydrogen could produce radiation at a frequency of 1 420.4058 MHz due to two closely spaced energy levels in the ground state of the hydrogen atom . The 21 cm line (1420.4 MHz) was first detected in 1951 by Ewen and Purcell at Harvard University, ...
What is the ground state of hydrogen?
The ground state of neutral hydrogen consists of an electron bound to a proton. Both the electron and the proton have intrinsic magnetic dipole moments ascribed to their spin, whose interaction results in a slight increase in energy when the spins are parallel, and a decrease when antiparallel. The fact that only parallel ...
What frequencies are the redshift lines?
Including the redshift, this line will be observed at frequencies from 200 MHz to about 9 MHz on Earth. It potentially has two applications.
What is the origin of the spectral lines in the hydrogen atom?
The origin of spectral lines in the hydrogen atom (Hydrogen Spectrum) can be explained on the basis of Bohr’s theory. The hydrogen atom is said to be stable when the electron present in it revolves around the nucleus in the first orbit having the principal quantum number n = 1. This orbit is called the ground state.
What does the horizontal line on a hydrogen atom mean?
Energy level diagrams indicate us the different series of lines observed in a spectrum of the hydrogen atom. The horizontal lines of the diagram indicate different energy levels. The vertical lines indicate the transition of an electron from a higher energy level to a lower energy level.
What is the characteristic x-ray emission?
Characteristic x-rays are emitted from heavy elements when their electrons make transitions between the lower atomic energy levels. The characteristic x-ray emission which is shown as two sharp peaks in the illustration at left occurs when vacancies are produced in the n=1 or K-shell of the atom and electrons drop down from above to fill the gap.
What are X-rays called?
For a particular material, the wavelength has definite value. Hence these x rays are called continuous or characteristic X-rays. The values of energy are different for different materials. The frequencies of the characteristic x-rays can be predicted from the Bohr model.
What is a common Bohr model picture?
Common pictures are those of a shell structure with each main shell associated with a value of the principal quantum number n. This Bohr model picture of the orbits has some usefulness for visualization so long as it is realized that the "orbits" and the "orbit radius" just represent the most probable values of a considerable range of values.
Does the Bohr model agree with the energy levels?
The energy levels agree with the earlier Bohr model, and agree with experiment within a small fraction of an electron volt. If you look at the hydrogen energy levels at extremely high resolution, you do find evidence of some other small effects on the energy. The 2p level is split into a pair of lines by the spin-orbit effect.

Overview
The emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to the electron making transitions between two energy levels in an atom. The classification of the series by the Rydberg formula was important in the development of quantum mechanics. The spectral series are i…
Physics
A hydrogen atom consists of an electron orbiting its nucleus. The electromagnetic force between the electron and the nuclear proton leads to a set of quantum states for the electron, each with its own energy. These states were visualized by the Bohr model of the hydrogen atom as being distinct orbits around the nucleus. Each energy level, or electron shell , or orbit, is designated …
Rydberg formula
The energy differences between levels in the Bohr model, and hence the wavelengths of emitted or absorbed photons, is given by the Rydberg formula:
where
Z is the atomic number, n′ (often written ) is the principal quantum number of the lower energy level, n (or ) is the principal quantum number of the upper energy level, and is the Rydberg consta…
Extension to other systems
The concepts of the Rydberg formula can be applied to any system with a single particle orbiting a nucleus, for example a He ion or a muonium exotic atom. The equation must be modified based on the system's Bohr radius; emissions will be of a similar character but at a different range of energies. The Pickering–Fowler series was originally attributed to an unknown form of hydrogen with half-integer transition levels by both Pickering and Fowler, but Bohr correctly recognised the…
See also
• Astronomical spectroscopy
• The hydrogen line (21 cm)
• Lamb shift
• Moseley's law
• Quantum optics
External links
• Spectral series of hydrogen animation