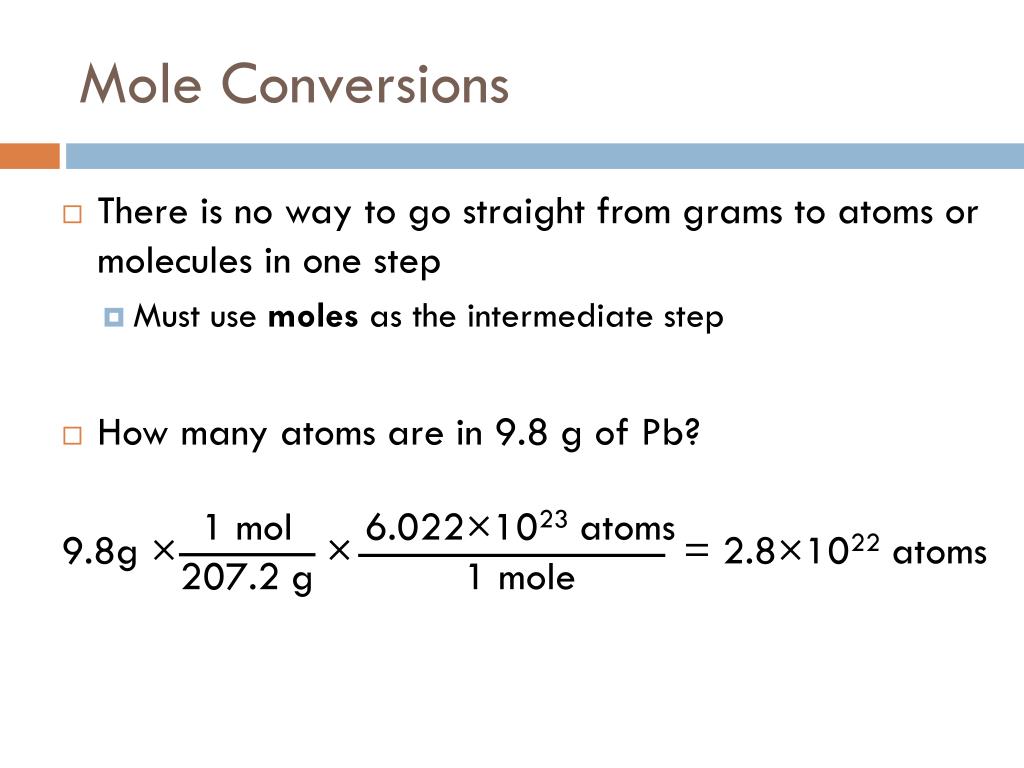
The simplest type of manipulation using molar mass as a conversion factor is a mole-mass conversion (or its reverse, a mass-mole conversion). In such a conversion, we use the molar mass of a substance as a conversion factor to convert mole units into mass units (or, conversely, mass units into mole units).
How to do mole conversions?
- From mass (grams) to moles: Divide your initial mass by the molar mass of the compound as determined by the periodic table. ...
- From volume (liters) to moles: Divide your initial volume by the molar volume constant, 22.4 L. ...
- From particles (atoms, molecules, or formula units) to moles: Divide your particle value by Avogadro’s number, 6.02 × 10 23. ...
How do you convert moles to mass?
To convert moles into mass, we first need to find the molar mass of the compound given. This can be done by looking at the mass of each element in the periodic table and then multiplying each mass by the number of atoms of that element in the compound. Molar mass has the units grams/mol which allows to convert between moles and grams.
How do you calculate mole ratio?
Unbalanced Equation Example
- The first step is to find how many moles of ozone are in 0.2 grams. ...
- To convert grams to moles, look up the atomic weight of oxygen on the periodic table. ...
- To find how many moles there are in 0.2 grams, solve for: x moles = 0.2 grams * (1 mole/16.00 grams). ...
How many mmoL are in a mol?
The mole (abbreviation mol) is the SI unit for the amount of a substance. A mmol (one thousandth of a mol) is a way to talk about a part of a mol. Thus, the conversion factor from mol to mmol is 1,000 mmol in 1 mol. Converting to mmol can be convenient if the number of mol is small.

How to convert moles to atoms?
So, to convert moles to atoms, we multiply by Avogadro’s number.
What can we use the number of moles to calculate?
We can also use the number of moles to calculate the number of atoms that are present.
How many moles of helium are in 8.0 grams?
So 8.0 grams of helium divided by 4.0 grams per mole gives us 2.0 moles of helium.
How many times is one mole of silver?
From Avogadro’s number, we know that one mole of silver atoms is equal to 6.02 times 10 to the 23rd silver atoms.
What is a mole in chemistry?
Moles are a counting unit that make it easier for chemists to work with large numbers.
Do grams cancel out moles?
Once again, grams cancel out to give moles.
What is the symbol for mol?
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is "mol."
What is the base unit of a substance?
The SI base unit for amount of substance is the mole.
What is a Mole?
The Mole is an amount unit similar to pair, dozen etc. and it contains 6.02214076 ∗ 10 23 number of particles, whereas this number ( 6.02214076 ∗ 10 23) is called the Avogadro’s Number or Avogadro’s Constant.Very large quantities of very small entities such as atoms, molecules or other specified particles are measured in Mole (also spelled as mol).
Why use mole calculator?
Use of this mole calculator comes in handy when you are solving some complex problem and don’t want to get involved in repetitive tasks. Due to their extremely small sizes, atoms, molecules and formula units are usually very difficult to work with. However, mole enables a chemist to work with large enough quantities which are handle able for practical uses.
How to find moles of a substance?
If we need to calculate the number of moles of a substance, simply divide the given Mass of the Substance by the given Molar Mass. This conversion can also be done using our grams to moles calculator.
Can you use grams to moles calculator?
These calculations can easily be replicated using our mass to moles calculator. Relatively complex problems involving large amounts of masses and molar masses can be solved instantly using this grams to moles calculator. It also has an added benefit of determining the number of constituent particles in each sample thus, it serves as a grams to molecules calculator as well.