So, if we include the nonmetals group, halogens, and noble gases, all of the elements that are nonmetals are:
- Hydrogen (sometimes)
- Carbon
- Nitrogen
- Oxygen
- Phosphorus
- Sulfur
- Selenium
- Fluorine
What are examples of non-metal elements?
Hydrogen, hydrogen, chlorine, fluorine, carbon, nitrogen, arsenic, phosphorus, selenium are examples of non-metal. What defines non-metal? A chemical element (such as boron, carbon or nitrogen) that lacks metal properties and is capable of forming anions, acid oxides, acids, and stable hydrogen compounds. What are non-metal and metal?
Which of the following elements is not a metal?
So, if we include the nonmetals group, halogens, and noble gases, all of the elements that are nonmetals are: Hydrogen (sometimes) Carbon. Nitrogen. Oxygen. Phosphorus.
How do you name nonmetals in chemistry?
Nonmetals can have names ending with -gen, - ine, or - on (hydrogen, oxygen, chlorine, argon). A metal's use is directly linked to its qualities. For example: Shiny metals such as copper, silver, and gold are often used for decorative arts, jewelry, and coins.
How many elements belong to the nonmetals group?
There are 7 elements that belong to the nonmetals group: Hydrogen (sometimes considered an alkali metal)

What are the 10 nonmetals?
List of All Elements That Are NonmetalsHydrogen (sometimes)Carbon.Nitrogen.Oxygen.Phosphorus.Sulfur.Selenium.Fluorine.More items...•
What are nonmetals?
Definition: The non-metals are elements on the right of the periodic table. Non-metals can be gases, liquids or solids. Non-metals are dull in colour, not shiny like metals. You can't hammer or shape a non-metal; it will just shatter if you hit it. Sulphur is an example of a non-metal.
What are the 7 nonmetals?
After the nonmetallic elements are classified as either noble gases, halogens or metalloids (following), the remaining seven nonmetals are hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur and selenium.
What are 22 nonmetals?
Seventeen elements are generally classified as nonmetals; most are gases (hydrogen, helium, nitrogen, oxygen, fluorine, neon, chlorine, argon, krypton, xenon and radon); one is a liquid (bromine); and a few are solids (carbon, phosphorus, sulfur, selenium, and iodine).
What are the 20 nonmetals?
Hydrogen, Helium, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Phosphorous, Sulphur, Chlorine, and Argon are the non-metals in the first twenty elements.
What are 3 nonmetals examples?
Non-metallic elements in the periodic table include hydrogen, carbon, nitrogen, oxygen, phosphorous, sulphur, silicon, boron, tellurium and selenium. They also include halogens (fluorine, chlorine, bromine, iodine and astatine) and noble gases (helium, neon, argon, krypton, xenon and radon).
What is non-metal Class 8?
Non – metals – Non- metals like coal and sulphur are soft and dull in appearance. They are not sonorous and are poor conductors of heat and electricity. Examples of non-metals are sulphur, carbon, oxygen, phosphorus, etc.
Is wood a non-metal?
Wood: While it seems obvious, wood is a non-metallic material that sees heavy use in the construction industry.
What are the 17 nonmetals?
Nonmetals are located on the far right side of the periodic table, except hydrogen, which is located in the top left corner. The 17 nonmetal elements are: hydrogen, helium, carbon, nitrogen, oxygen, fluorine, neon, phosphorus, sulfur, chlorine, argon, selenium, bromine, krypton, iodine, xenon, and radon.
Is plastic non-metal?
Is plastic non-metal? For elements, the term metal and non-metals are used. Plastic is not an element but a polymer consisting of various non-metals such as carbon, hydrogen, oxygen, nitrogen and so on.
Is Diamond a metal?
Diamond is not a metal in anyway its just an allotrope of carbon. It does not show any physical properties or chemical properties of metals like electrical conductivity, malleability, ductility, reaction with acids or salts etc.
What are non-metals for Class 6?
Examples of non-metals: Carbon, nitrogen, oxygen, phosphorous and sulphur.
What are the 18 nonmetals in periodic table?
Periodic Table Metals and Nonmetals. In the above table nonmetal elements are H,He,C,N,O,F,Ne,P,S,Cl,Ar,Se,Br,Kr,I,Xe,At and Rn.
Is carbon a nonmetal?
Carbon is a true nonmetal in every sense. Lead is a true metal. Silicon is almost completely nonmetallic; tin is almost completely metallic.
What are the 10 example of metal?
Examples of metals are aluminium, copper, iron, tin, gold, lead, silver, titanium, uranium, and zinc. Well-known alloys include bronze and steel. The study of metals is called metallurgy.
Is boron a non-metal?
Boron is a non metallic element and the only non-metal of the group 13 of the periodic table the elements. Boron is electron-deficient, possessing a vacant p-orbital. It has several forms, the most common of which is amorphous boron, a dark powder, unreactive to oxygen, water, acids and alkalis.
Is silicon a non-metal?
Silicon is neither metal nor non-metal; it's a metalloid, an element that falls somewhere between the two.
Is oxygen a nonmetal?
oxygen (O), nonmetallic chemical element of Group 16 (VIa, or the oxygen group) of the periodic table.
Is balloon a non metal?
Other nonmetal gases include hydrogen, fluorine, chlorine, and all the group eighteen noble (or inert) gases. Helium is chemically non-reactive, so it is useful for applications such as balloons (see figure below) and lasers, where non-flammability is extremely important.
What is the 10 metal?
The metals list which makes up the periodic table includes iron, lead, gold, aluminum, platinum, uranium, zinc, lithium, sodium, tin, silver, etc. The nonmetals list which makes up the periodic table includes hydrogen, helium, carbon, sulfur, nitrogen, oxygen, radon, neon, other halogens, and noble gases etc.
What is difference between metals and nonmetals?
Metals are good conductors of heat and electricity. Non-metals are bad conductors of heat and electricity. Except for graphite which is a good conduction of electricity. Metals are lustrous and can be polished.
What are non-metals and their properties?
Nonmetals are typically poor conductors of heat and electricity, with low melting and boiling points. They tend not to be malleable or ductile, so they form brittle solids. Nonmetals include solids, liquids, and gases at room temperature and pressure.
Which is an example of a nonmetal *?
Helium is one of the many nonmetals that is a gas. Other nonmetal gases include hydrogen, fluorine, chlorine, and all the group eighteen noble (or inert) gases.
What are the 5 properties of non-metals?
Five physical properties of non-metals are:Non-metals are brittle. ... Non-metals are bad conductors of heat and electricity (except graphite).Non-metals are non-lustrous (dull) and cannot be polished (except iodine).Non-metals may be solids, liquids or gases at room temperature.Non-metals are neither tough nor strong.
What are examples of non-metals?
Hydrogen, chlorine, fluorine, carbon, nitrogen, phosphorus, selenium are examples of non-metal.
What defines non-metal?
A chemical element (such as boron, carbon or nitrogen) that lacks metal properties and is capable of forming anions, acid oxides, acids, and stable...
What are non-metal and metal?
Non-metals are such elements which have 4,5, 6 and 7 electrons in their outermost shell. Examples of non-metals are carbon, oxygen chlorine etc. M...
What are non-metallic materials?
Non-metals are natural materials that do not produce heat or electricity and that are structurally brittle (can not be easily rolling, moulding, ex...
What is called ductility?
Ductility is a measure of the ability of a metal to withstand tensile stress — any force that separates the two ends of an object from each other....
Is plastic non-metal?
For elements, the term metal and non-metals are used. Plastic is not an element but a polymer consisting of various non-metals such as carbon, hydr...
What is a liquid metal called?
Liquid metal is composed of alloys with very low melting points that form a liquid eutectic at room temperature. The main metal used to be mercury,...
How to tell if an element is metal or nonmetal?
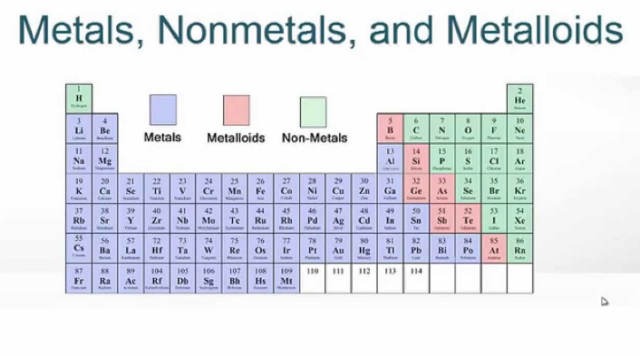
The easiest way to identify whether an element is a metal or nonmetal is to find its position on the periodic table. A zigzag line runs down the right side of the table. Elements on this line are metalloids or semimetals, which have properties intermediate between those of metals and nonmetals.
What are metals used for?
Uses for Metals and Nonmetals. A metal's use is directly linked to its qualities. For example: Shiny metals such as copper, silver, and gold are often used for decorative arts, jewelry, and coins. Strong metals such as iron and metal alloys such as stainless steel are used to build structures, ships, and vehicles including cars, trains, and trucks.
What are the qualities of metals?
A metal's use is directly linked to its qualities. For example: 1 Shiny metals such as copper, silver, and gold are often used for decorative arts, jewelry, and coins. 2 Strong metals such as iron and metal alloys such as stainless steel are used to build structures, ships, and vehicles including cars, trains, and trucks. 3 Some metals have specific qualities that dictate their use. For example, copper is a good choice for wiring because it is particularly good at conducting electricity. Tungsten is used for the filaments of light bulbs because it glows white-hot without melting.
What is sulfuric acid used for?
Sulfuric acid is an important tool for industry, used in batteries and manufacturing. Chlorine is a powerful disinfectant. It is used to purify water for drinking and fill swimming pools. Helmenstine, Anne Marie, Ph.D. "Examples and Uses of Metals and Nonmetals.".
What percentage of elements are metals?
The two rows of elements below the body of the periodic table are also metals. Basically, about 75% of elements are metals, so if you're given an unknown element and asked to make a guess, go with a metal. Element names can be a clue too. Many metals have names ending with -ium (e.g. beryllium, titanium).
Where are nonmetals on the periodic table?
The nonmetals are on the upper right-hand side of the periodic table. Nonmetals are typically poor electrical and thermal conductors and don't have a metallic luster. They can be found as solids, liquids, or gases under ordinary conditions. Examples include: Nitrogen.
Why is sulfur important to humans?
Not only do we breathe it and use it for medical purposes, but we also use it as an important element in combustion. Sulfur is valued for its medical properties and as an important ingredient in many chemical solutions.
How many elements are in the nonmetal group?
There are 7 elements that belong to the nonmetals group: Although these are the elements in the group nonmetals, there are two additional element groups that could be included, since the halogens and noble gases also are types of nonmetals.
What are the oxidation numbers of nonmetals?
Atoms of these elements have oxidation numbers of +/- 4, -3, and -2.
Where are nonmetals on the periodic table?
Updated February 25, 2020. The nonmetals or non-metals are a group of elements located on the right side of the periodic table ( except for hydrogen, which is on the top left). These elements are distinctive in that they typically have low melting and boiling points, don't conduct heat or electricity very well, and tend to have high ionization ...
Is carbon a nonmetal?
Nonmetals are classified based on their properties under ordinary conditions. Metallic character isn't an all-or-nothing property. Carbon, for example, has allotropes that behave more like metals than nonmetals. Sometimes this element is considered to be a metalloid rather than a nonmetal.
Why are nonmetals so variable?
This is because nonmetals differ widely in interatomic and intermolecular bonding strengths. Most nonmetals tend to be gasses at standard temperatures and pressure and have low densities.
Why do nonmetals have high ionization energies?
Nonmetals have high ionization energies because of how large their nuclei are compared to how full their electron shells are. The large, positively charged nuclei of atoms like oxygen and fluorine attract their electrons very strongly , making them difficult to remove.
Why do nonmetal compounds have low melting points?
Nonmetal compounds, specifically covalent compounds, have low melting and boiling points because of their relatively weak intermolecular interactions. The phase behavior of a substance is determined by the strength of its intermolecular bonds. Metals have very high melting and boiling points because they have very strong intermolecular attractions. Covalent compounds, on the other hand, do not have strong intermolecular attractions. This is because most covalent molecules are electrically neutral and so don’t attract their neighbors, at least, not to the extent that metals do.
Why are nonmetal compounds brittle?
Nonmetal compounds tend to be brittle on account of the nature of their ionic and covalent bonds. Both ionic and covalent bonds involve the sharing/capture of electrons. Both kinds of compounds arrange themselves in such a way as to minimize electrostatic repulsions. For example, in an ionic compound, positive and negative atoms are locked into place in a tight crystal structure with positive ions aligned to negative ions. Applying a force can shift the alignment of ions so that positives align with positives and negatives with negatives. The repulsion causes the compound to fracture.
What are nonmetals in chemistry?
In chemistry, the term “nonmetal” refers to elements and compounds that lack a metallic character. Despite making up only 17 of the 118 known elements, nonmetals are some of the most important elements that are essential for life as we know it. Examples of non-metals include carbon, oxygen, nitrogen, and hydrogen;
What are the two types of nonmetals?
Kinds Of Nonmetals. Generally, nonmetals are split into two categories: reactive nonmetals and halogens. Reactive nonmetals tend to show more variation in their physical and chemical properties. Some, like carbon and sulfur, are solid at room temperature and are less electronegative. Others, like oxygen, are a gas at room temperature ...
What is a nonmetal?
To sum up, a nonmetal is a chemical that is characterized by a lack of metallic properties. Nonmetals are typically gaseous or liquid at room temperature and are divided between the reactive nonmetals and noble gases.
Selenium (Se)
Selenium is a chemical element with the atomic number 34 in the periodic table. It occurs naturally in Earth’s crust, especially in carbonate rocks and volcanic and sedimentary soils. Being a member of the sulphur group of periodic table elements, this chalcogen has six electrons in the outermost shell and valences of -2, 4, and … Read more
Sulfur
Sulfur (S) Introduction Sulfur is a chemical element with the atomic number 16 in the periodic table. It’s the 5th most abundant element in Earth’s crust with an approximate concentration of 0.06 percent.
Phosphorus
Phosphorus (P) Introduction Phosphorus is a chemical element with the atomic number 15 in the periodic table. It is the eleventh most abundant element in Earth’s crust, with an occurrence of about 0.1% ppm. Due to its high reactivity, phosphorus does not occur freely in nature. Being a member of the nitrogen family of periodic … Read more
Oxygen (O)
Oxygen is a nonmetallic chemical element with atomic number 8 in the periodic table. Occurring with 467,100 ppm or 46% in Earth’s crust, it’s the most abundant element both in the thin outer layer of our planet and the human body. As a member of the oxygen family of periodic table elements, this chalcogen … Read more
Nitrogen (N)
Nitrogen (Azote) is a chemical element with atomic number 7 in the periodic table. It’s the most abundant element on Earth. Nitrogen naturally occurs in the Earth’s atmosphere, crust, mantle, core, as well as in oceans. In Earth’s crust, this substance is present in the form of a liquid metal, while in the atmosphere nitrogen … Read more
Carbon
Carbon is a chemical element with an atomic number of 6 in the periodic table of elements. It’s the 19th most abundant element found in nature. With a quantity of approximately 0.025 percent in Earth’s crust (2.00×102 milligrams per kilogram), carbon is not really plentiful. However, it is widespread and it’s one of the chemical … Read more
Hydrogen
Introduction Hydrogen is a chemical element with an atomic number of 1 in the periodic table. Occurring as a part of the H2O molecule, it’s the most plentiful element that occurs in the Universe. With 0.15 % concentration, it’s also the 10th most abundant chemical element in Earth’s crust. As a member of the hydrogen … Read more
Core Concepts
In this tutorial, you will be introduced to metals and non-metals on the periodic table of elements and their properties.
Metals
A majority of the elements on the periodic table of elements categorize themselves as metals. On the periodic table metals, are placed to the left of the zigzag line that runs between the five elements: boron, silicon, arsenic, tellurium, and astatine.
Examples of Metals
Some well-known metals on the periodic table include: iron, lead, aluminum, silver, calcium, and sodium which present as solids at room temperature. Mercury, which is classified as a metal, is the only metal on the periodic table that presents as a liquid . Out of the one hundred and eighteen metals on the periodic table gold is the most malleable.
Properties of Metals
All metals differ in their melting points, but generally, all metals have high melting and boiling points.
Metals and their Charges
Since the metals lie to the left of the periodic table they often have low ionization energies and low electron affinities, meaning they give away electrons relatively easy causing metals to become cations. The main group metals usually form charges that are the same as their group number.
Non-Metals
Non-Metals account for a small portion of elements on the periodic table. On the periodic table, non-metals lie to the right of the zigzag line that runs between the elements boron, silicon, arsenic, tellurium, and astatine. Non-Metals also include hydrogen which lies to the left of the metalloids. Many of these elements have biological roles.
Examples of Non-Metals
While there are only seventeen non-metals on the periodic table a few common examples include oxygen and nitrogen which account for most of the air that we breathe, along with a few other gases like neon or the chemical compound carbon dioxide.
What are the Characteristics of Nonmetals?
Non-metallic atoms are much smaller in size as compared to metallic atoms.
What are nonmetals used for?
Ans: Nonmetals are used widely in our daily lives. We breathe in oxygen for our survival which is non-metal. Nitrogen is of vital importance to plant physiology and agriculture. Noble gases like argon, xenon are used to construct glowing sign boards which are used for advertisement purposes. Chlorine, a nonmetal, is used for the purification of drinking water. Iodine is used as an antiseptic for application on the wound. It prevents microbial growth on the wound. It is also used for the preparation of anti-bacterial gurgles for the production of throat Infection. Carbon, a nonmetal is an element around which the whole of the organic world is composed of. Carbon is the constituent of every organic compound. Carbon is also used for manufacturing carbon fibre which is used for making fishing rods, rockets, aeroplanes, etc.
What is iodine used for?
Iodine is used as an antiseptic for application on the wound. It prevents microbial growth on the wound. It is also used for the preparation of anti-bacterial gurgles for the production of throat Infection. Carbon, a nonmetal is an element around which the whole of the organic world is composed of.
What are nonmetals in the periodic table?
These elements are located at the upper right corner of the modern periodic table. Examples of nonmetals are nitrogen, carbon, oxygen, hydrogen, sulphur, etc. At room temperature, most of the nonmetals are gases or solids. The only liquid non-metal at room temperature is Bromine. They are good insulators of heat and electricity with an exception ...
Why are nonmetals important?
nonmetals are of vital importance to mankind. They are necessary for sustaining life on earth. There are varied uses of nonmetals in various fields. These nonmetals are also necessary for the biological system. Most of these nonmetals are present in the human body in certain percentages.
How many electrons are in a nonmetal?
Nonmetals can be defined as the elements that form negative ions by accepting electrons from other elements. They usually have 4,5,6 or 7 electrons in their valence shell. nonmetals lack metallic characteristics.
What is nitrogen used for?
Nitrogen is used for making nitrogenous fertilizers which are essential for plant growth. Nitrogen is an essential nutrient for plant growth. Nitrogen is also used in food packaging.
What are the main properties of nonmetals?
Physical Properties of Nonmetals Non-Malleable and Ductile: Non-metals are very brittle, and cannot be rolled into wires or pounded into sheets. Conduction: They are poor conductors of heat and electricity. Luster: These have no metallic luster and do not reflect light.
What are the 10 characteristics of non metals?
Characteristics of Non-metals 1)Non-Metals are not malleable. 2)Non-Metals are not ductile. 3)They are bad conductor of heat and electricity. 4)They are not lustrous or shiny.
What are two properties of most nonmetals?
What are two properties of most nonmetals? 1) high ionization energy and poor electrical conductivity 2) high ionization energy and good electrical conductivity 3) low ionization energy and poor electrical conductivity 4) low ionization energy and good electrical conductivity 13.
How are nonmetals different from metals?
Non- metals are brittle (break easily). They are neither malleable nor ductile. Metals are good conductors of heat and electricity. Non-metals are bad conductors of heat and electricity.
Which nonmetals have similar properties?
What are the similar properties of nonmetals? Nonmetals share many similar properties including: They are either gas (hydrogen, oxygen, nitrogen) or solid (carbon, sulfur) under standard conditions. They are not good conductors of electricity or heat.
What are 3 properties of non – metals?
Properties of Non-metals. The element which has the tendency to take negative electrons by accepting electrons is called non-metal. That is, electricity is negative in non-metal. Such as carbon, nitrogen, sulfur, etc. Non-metals are found in all three states: solid, liquid, and gas.
