What is organic surface active agents?
- Soaps (free fatty acid salts)
- Fatty acid sulfonates (the most common of which is sodium laryl sulfate, or SLS)
- Ethoxylated compounds, such as ethoxylated propylene glycol.
- Lecithin.
- Polygluconates, basically a glorified name for short-chain starches.
What is a surface-active agent?
Jan 08, 2020 · What are organic surface active agents? SURFACE ACTIVE AGENTS. A surface active agent (= surfactant) is a substance which lowers the surface tension of the medium in which it is dissolved, and/or the interfacial tension with other phases, and, accordingly, is positively adsorbed at the liquid/vapour and/or at other interfaces.
How do surface-active agents self-assemble?
Jan 11, 2020 · What is organic surface active agents? Soaps (free fatty acid salts) Fatty acid sulfonates (the most common of which is sodium laryl sulfate, or SLS) Ethoxylated compounds, such as ethoxylated propylene glycol. Lecithin. Polygluconates, basically a glorified name for short-chain starches.
What are surface-active agents in the dyeing of textiles?
Surface active agents, also known as tensides, amphiphiles or surfactants (for short) is a general name for substances that tend to preferentially accumulate at the boundary (i.e., interface) between two phases. This adsorption at the various interfaces existing between solids, liquids, and gases causes a change in the nature of interfaces which are of considerable importance in …
What are surface-active molecules?
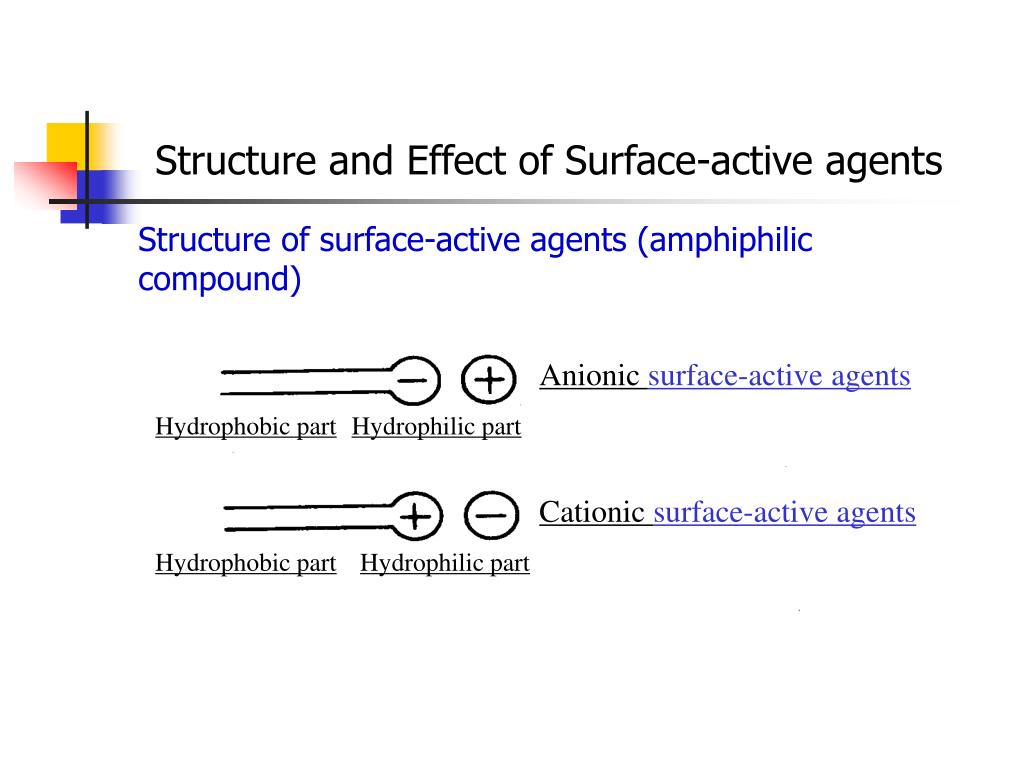
Surface active agents (surfactants) are amphiphilic compounds with two opposing portions, one part is hydrophilic and the other is hydrophobic [ 1 ]. They reduce the free energy of the system by replacing the bulk molecules of higher energy at an interface.
What are surface-active agents examples?
What are the 4 types of surfactants?
- Anionic Surfactants. Anionic surfactants have a negative charge on their hydrophilic end. ...
- Nonionic Surfactants. Nonionic surfactants are neutral, they do not have any charge on their hydrophilic end. ...
- Cationic Surfactants. ...
- Amphoteric Surfactants.
What are synthetic organic surface-active agents?
What are surface-active agents used for?
What are natural surfactants?
What is surfactant in pharmaceutical?
What does anionic and nonionic mean?
What is the difference between ionic and nonionic surfactants?
What is the difference between ionic and nonionic?
What is meant by surface activity?
How do surface active agents reduce surface tension?
Is surfactant a medication?
What is surface active agent?
Surface active agents, also known as tensides, amphiphiles or surfactants (for short) is a general name for substances that tend to preferentially accumulate at the boundary (i.e., interface) between two phases. This adsorption at the various interfaces existing between solids, liquids, and gases causes a change in the nature of interfaces which are of considerable importance in pharmacy. Thus, the lowering of the interfacial tension between oil and water phases facilitates emulsion formation, the adsorption of surfactants on insoluble particles enables these particles to be dispersed in the form of a suspension, their adsorption on solid surfaces enables these surfaces to be more readily wetted, and the incorporation of insoluble compounds within micelles of the surfactant can lead to the production of clear solutions.
What is anionic surfactant used for?
Consequently, anionic surfactants are used most widely and extensively in detergents, shampoos, and body cleansers.
What is the difference between anionic and anionic surfactants?
The anionic surfactants adsorb on various kinds of substrates and give them an anionic charge. This action of the anionic surfactant contributes to the strong detergency and high foaming power of the agent. Consequently, anionic surfactants are used most widely and extensively in detergents, shampoos, and body cleansers.
How do cationic surfactants adsorb?
Most materials have negative charges in an aqueous media, and the cationic surfactant molecules adsorb by orienting their hydrophilic head group toward the surface of the materials. This characteristic of the cationic surfactant is fully utilized in several products such as fabric softeners and hair conditioners.
What are the different types of surfactants?
For pharmaceutical purposes, surfactants are most satisfactory classified by means of the type of polar group into; Anionic surfactants. Cationic surfactants. Amphoteric Surfactants. Non-ionic Surfactants.
What is an amphoteric surfactant?
Amphoteric surfactants. Amphoteric or ampholytic surfactants sometimes referred to as zwitterionic molecules are surface-active agents that possess both cationic and anionic groups in the same molecule. Their ionization state in solution depends on the pH of the medium and the pKa of ionizable groups. For example, the acidic functional groups, such ...
What are the two regions of a molecule called?
The existence of such two regions in a molecule is known as amphipathy, and the molecules are consequently often referred to as amphiphiles or amphipathic molecules. Schematic representation of surfactant. The hydrophobic portions are usually saturated or unsaturated hydrocarbon chains or, less commonly, heterocyclic or aromatic ring systems ...
What is the role of surface active substances in the ocean?
A surface active substance (SAS) (also known as a surfactant) plays an important role at the air–sea interface both in terms of enrichment of matter and as binder of organic and inorganic species. Biogenic surfactants, whose principal source is attributed to the phytoplankton population, greatly influence the energy and mass transfer between the boundary layers, playing an important role in biogeochemical cycles, in the distribution and fate of pollutants, and in air–sea gas exchange processes. Even if considered as refractory organic matter, humic-like substances (HULIS or HS) could also be considered as SASs.
What is a surfactant?
Surfactants are sur face- act ive ag ents that aggregate near or have a strong effect on modifying the interface between two materials. This occurs because of their dual nature: hydrophobic and hydrophilic. The hydrophilic moiety may be anionic (carboxylate, sulfate, or phosphate), cationic (quaternary ammonium salt), ampholytic (cationic and anionic), or nonionic (Span, Tween, Triton) depending on the type of charge (s) carried, if any. Performance of surfactants depends strongly on their surface activity and micelle formation.
What are the different types of surfactant structures?
Figure 5. Surfactant self-assembly leads to a range of different structures of which a few are shown: (a) Spherical micelle with an interior composed of the hydrocarbon chains and a surface of the polar head-groups (depicted as spheres) facing water. The hydrocarbon core has a radius close to the length of the extended alkyl chain. (b) Cylindrical micelle with an interior composed of the hydrocarbon chains and a surface of the polar head-groups facing water. The cross section of the hydrocarbon core is similar to that of spherical micelles. The micellar length is highly variable so these micelles are polydisperse. (c) Lamellar phase consisting of surfactant bilayers. (d) Reverse micelle with a water, core surrounded by the surfactant polar head-groups. The alkyl chains together with a non-polar solvent make up the continuous medium. (e) Bicontinuous structure with the surfactant molecules assembled into connected films characterized of two curvatures of opposite sign. (f) Vesicle built from bilayers similar to those of the lamellar phase, and characterized by two distinct aqueous domains, one forming the core and one the external medium.
How do amphiphilic molecules interact?
Due to the amphiphilic nature of a surfactant or lipid, the two parts of the same molecule interact very differently with either a polar solvent or surface or a nonpolar solvent or surface. There are two different ways to render favorable intermolecular contacts possible in surfactants while eliminating unfavorable ones: self-assembly in solution (see Figure 1) and adsorption at a surface or an interface (examples shown in Figure 2 ). The self-assemblies can involve only the amphiphilic molecules, or there can be a mixed aggregate formed together with a low molecular weight cosolute or a macromolecule [ 2 ]; in fact, self-assembly is quite sensitive to a range of cosolutes. During the recent years, we have learnt that amphiphile adsorption at different interfaces is also best considered as a self-assembly process promoted by the interface and that there is considerable organisation of amphiphilic molecules at interfaces.
What are tensides in water?
Tensides are surface-active substances, which by reducing the surface energy of water mediate the wetting of surfaces or promote that substances insoluble in water can be emulgated or dispersed in it.
Can surfactant molecules self-assemble?
Figure 2. Surfactant molecules can self-assemble into discrete (spherical or cylindrical) micelles at a hydrophilic surface, in the absence of strong specific interactions between the surfactant head-group and the surface.
Is surfactant cationic or ionic?
Other complexities in surfactant structure also commonly arise. Many surfactants, both ionic and nonionic, contain multiple hydrophilic groups, the multiplicity itself imparting special properties. As important and growing class of surfactants consists of ionics (either anionic or cationic) which are polyoxyethylated to various extents. These compounds have significantly different properties from the simple ionics.
What is surface active agent?
Surface active agents are commonly used in cell culture to prevent cells from adhering to gas-liquid interfaces, to solubilize hydrophobic nutrients, to minimize foaming in sparged cultures, and to inhibit cell clumping.
What are surfactants used for?
Surfactant are surface active agents, which reduces surface tension of liquids or interfacial tension between a multiple-phase system (usually a two-phase system) by adsorbing on the surface/interface. The role of surfactants in the protein-based formulation is to block the protein's interaction with hydrophobic surfaces (e.g., vial walls or air interfaces), which otherwise can cause denaturation and aggregation of proteins. Some biologics products need surfactants to block the particular subregions of the protein or to block the protein-protein interaction that could lead to denaturation. The surfactants like polysorbate (Polysorbate 80 or Polysorbate 20) and Poloxamer 188 (PO188) are mainly used in protein-based biologics formulation. Even in the presence of these surfactants, protein drug stability is often insufficient, particularly because of agitation-induced aggregation. In recent reports, N-myristoyl phenylalanine Jeffamide (FM1000) has been proven to have better interfaces blocking capacity and stabilizes faster than other conventional surfactants.
How does surfactant affect nanofluids?
Surfactants are used to stabilize nanofluids, and also influence the surface tension of nanofluids. The effect of surfactant on the surface tension of nanofluid is shown in Fig. 4-51. It can be seen from Fig. 4-51 that surface tension decreases with an increase in the concentration of surfactant. Kumar and Malinova (2009) studied the effect of surfactant on the surface tension of SWCNT–water nanofluids with NaBDS surfactant and found that in the case of water and surfactants, surface tension initially decreased gradually 0.62 vol.% and became constant for 0.62–1.30 vol.%. However, when the SWCNT was added to DIW with surfactant, the surface tension was the same for low volume fraction (0.05–0.2 vol.%). After this range, it gradually decreased from 0.25 to 1.3 vol.% as most of the surfactant molecule was mixed with the DIW. Similarly, in the case of n-decane, 0.1 wt.% of Al/surfactant (sorbitan oleate) mixture showed that with an increase in the volume fractions of surfactant, the resulting surface tension of nanofluid reduced, as shown in Fig. 4-52. As the long-chain surfactant molecules attached to the solid particle, they form a layer between the particle and the surrounding fluid molecules. Such layers increase the potential between particles and impart a repulsive force between them. This, in turn, causes a reduction in surface tension ( Tanvir and Qiao, 2012 ). [This paragraph is adapted from Khaleduzzaman et al. (2013), copyright (2013), with permission from Elsevier.]
What are some examples of surfactants?
Examples include sugar ester surfactants (e.g., sorbitan monooleate), polyoxyethylene ether surfactants such as Brij 96 (used in detergent industry), and ethoxylated sorbitan esters (polysorbates) such as Tween 60 (polyoxyethylene sorbitan monostearate) and Tween 80 (polyoxyethylene sorbitan monooleate) [13]. Mixtures of non-ionic surfactants are far more effective than the other class of surfactants for ME formation as these potentially eliminate the need for a co-surfactant.
How do surfactants affect particle removal?
Surfactants can enhance particle removal from surfaces by modifying the particle-surface interaction forces. Adsorbed surfactant molecules can alter the van der Waals attractive force, electrostatic force, hydrophobic force, as well as provide a steric barrier to contact. The effect of surfactants on these forces can result in greatly enhanced particle removal efficiency.
Why do T-shape surfactants work better than linear surfactants?
T-shape surfactants work better than flexible linear surfactants because they facilitate the formation of a liquid film. As shown in Figure 3.80, at the bottom left corner, T-shape molecules rearrange between the liquid interface and the solid interface to form a “sandwich” laminating a water film.
What are the building blocks of SLNs?
Surfactants, surface-active agents, are the other important building blocks of the SLNs systems. Surfactants are amphiphilic compounds that possess a hydrophilic polar moiety and a lipophilic nonpolar moiety. These together constitute the typical head and the tail of surfactants. When used in low concentrations, surfactants adsorb onto the surface or interface of a system. They reduce the surface or interfacial free energy, thus ultimately leads to reduction in surface or interfacial tension between two phases ( Shah et al., 2015a ).
Latest Trends
The following visualization shows the latest trends on Organic surface-active agents, nes. Countries are shown based on data availability.
Historical Data
Organic surface-active agents, nes are the world's 2020th most traded product.
Tariffs
In 2018, the average tariff for importing Organic surface-active agents, nes was 7.2%. The countries with the highest tariffs for importing Organic surface-active agents, nes were Bahamas (40.2%), Laos (38.2%), Sudan (35%), and Fiji (31.1%).
Product Complexity
The Complexity-Relatedness diagram compares the risk and the strategic value of a product's potential export opportunities. Relatedness is predictive of the probability that a country increases its exports in a product. Complexity, is associated with higher levels of income, economic growth potential, lower income inequality, and lower emissions.
What are surface active agents?
Also known as surfactants, surface-active agents are basic cleaning agents in soaps and detergents. These agents are added to wash water to lower its surface tension, thereby to increase the wetting and spreading properties of water. Surfactants are usually organic compounds, which are amphiphilic, meaning they are soluble in both organic solvents and water. Surface active agents have two parts, one is hydrophilic (water loving) and another is hydrophobic (water repellent). Surface-active molecules concentrate at the areas of contact or interfaces, between oil and water. One end of the molecule seeks oil, while the other end seeks water. At the interface of water and oil, surface-active agents emulsify oil and mix it into the liquid in the same way fat is mixed in milk. At the interface of water, these agents trap air molecules to produce foam.
What are the different types of surface active agents?
On the basis of ionic properties, surfactants can be classified into four types -. Anionic Surfactants. Non-ionic Surfactants. Cationic Surfactants.
How many surfactants are in a micelle?
There is typically between a few dozen to a couple of hundred surfactant molecules in a micelle.
What aggregates to develop and produce extended structures in water like that of surfactant bilayers?
Surfactants also aggregate to develop and produce extended structures in water like that of surfactant bilayers.
How do surfactants help clean?
By reducing the surface tension water, surfactants improve the cleaning performance by enabling the solution to wet a surface (for example , dishes, clothes, and countertops) quickly and effectively , and hence the soil can be readily loosened and removed.
What is the process of a surfactant adsorbing?
Adsorption is the tendency of the molecule of a surfactant to collect as an interface. It is the taking up of a liquid or gas at the surface of substance, generally a solid (for example, activated charcoal adsorbs gases). The process involves molecular attraction at the surface.
What is affinity surfactant used for?
Affinity surfactants have found uses as reversibly bound ligands in high performance affinity chromatography
What is surface active agent?
Surface-active agents are one of the most commonly applied compounds in industrial, agricultural, and household activities , and after use a huge number of surface active agents (and/or their degradation products) are discarded to wastewater-treatment plants.
What is the classification of surface active agents?
Classification of surface active agents is based on the charge of hydrophilic parts of their molecules: Anionic - Based on permanent anions or pH-dependent anions. Cationic - Based on pH-dependent primary, secondary or tertiary amines. Non-ionic - Does not include any charge head.
Is a surface active molecule hydrophilic or lipophilic?
The surface-active molecule must be partly hydrophilic (water-soluble) and partly lipophilic (soluble in lipids, or oils). It concentrates at the interfaces between bodies or droplets of water and those of oil, or lipids, to act as an emulsifying agent, or foaming agent.