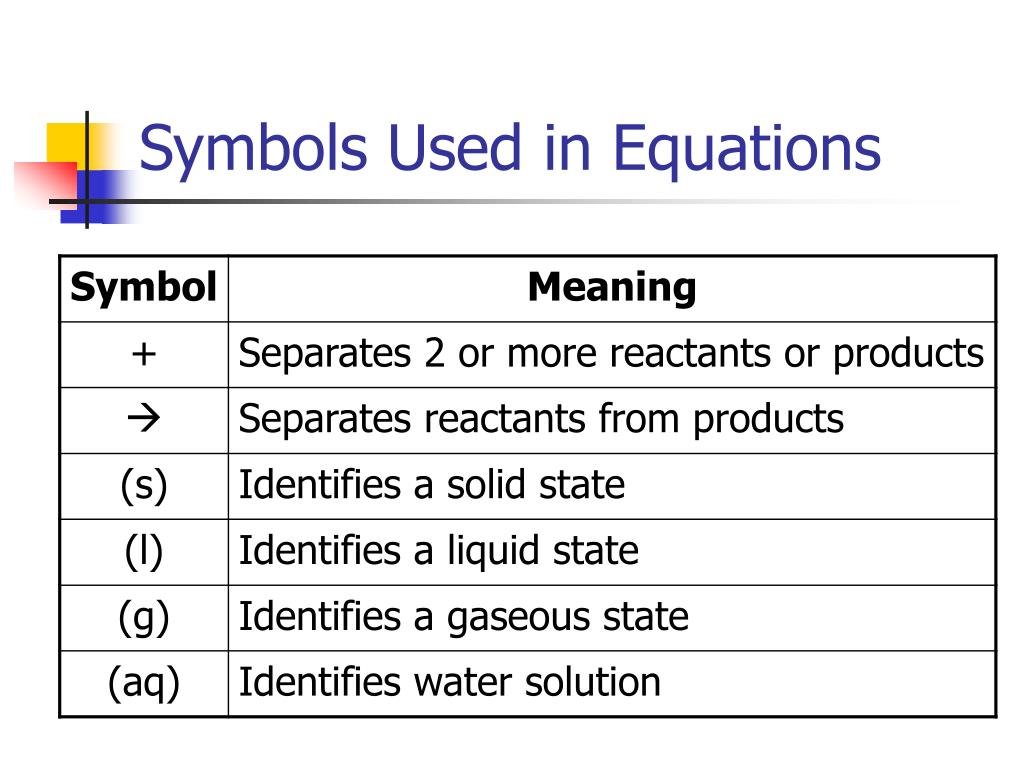
| Symbol | Meaning |
|---|---|
| (s) | indicates that the substance is in a solid state |
| an alternative way of representing a substance in a solid state | |
| (aq) | indicates that the substance is dissolved in water - the aq comes from aqueous |
| indicates that heat is applied to make the reaction proceed |
What are state symbols in a chemical reaction?
Using state symbols in chemical equationsState symbolMeaning(s)solid(l)liquid(g)gas(aq)aqueous (dissolved in water)
Where are state symbols in a chemical equation?
1:343:36State Symbols in Chemical Equations | Properties of Matter | FuseSchoolYouTubeStart of suggested clipEnd of suggested clipAnd product there are four possible states each with their own unique state symbol solid representedMoreAnd product there are four possible states each with their own unique state symbol solid represented by lowercase s liquid represented by lowercase l gas represented by lowercase g.
What are the states of chemical equation?
These states are:Solid, given the symbol (s)Liquid, given the symbol (l)Gas, given the symbol (g)Aqueous, meaning dissolved in water, and given the symbol (aq)
Should state symbols be subscript?
Symbols of State To indicate the states of the products and reactants in a reaction, we write the following symbols as subscripts after each chemical in the equation. Sometimes it's easy to tell what symbols of state should be used, and sometimes it's not. For example, water is frequently in a liquid form.
What is the state symbol for H2O?
H 2O(l) is liquid water but H 2O(g) is steam....State symbols.State symbolMeaning(g)Gas(aq)Aqueous solution2 more rows
Are states written as subscripts?
Explanation: In a chemical equation, the three states of matter are expressed as (g) for gas, (s) for solid, and (l) as liquid. They should be written as a subscript after the chemical formula, but that has become less common.
What does the state symbol g mean?
gaseous state(g) indicates that the substance is in a gaseous state. an alternative way of representing a substance in a gaseous state. (s) indicates that the substance is in a solid state.
What is the symbol of solid?
Step 1. The symbols used to represent gases, liquids, solids and aqueous phases are (g), (l), (s), and.
What does the state symbol in brackets mean?
The state symbols in brackets show the physical state of. the substance at the reaction temperature. Solid (s), liquid (l), gas (g), or dissolved in water (aq). aq is called aqueous which comes. from the Latin word aqua meaning water. If you do not know the state of a substance. see melting and boiling points.
What are the S tate symbols?
S tate Symbols used in Chemical Equations. still require state symbols to be complete. potassium + chlorine potassium chloride. lith ium + oxygen lithium oxide. the substance at the reaction temperature. Solid (s), liquid (l), gas (g), or dissolved in water (aq). from the Latin word aqua meaning water.
What is the substance if the melting point is below the reaction temperature?
If the melting point is above the reaction temperature, the substance is solid (s). If the boiling point is below the reaction temperature, the substance is gas (g). If the melting point is below the reaction temperature. and the boiling point is above the reaction temperature, then the substance is liquid (l).