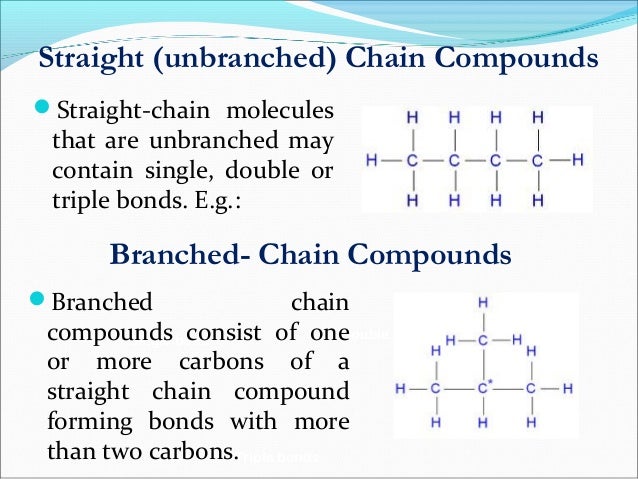
What are the 4 types of bonding?
- Ionic bond. Ionic bonding involves a transfer of an electron, so one atom gains an electron while one atom loses an electron.
- Covalent bond. The most common bond in organic molecules, a covalent bond involves the sharing of electrons between two atoms.
- Polar bond.
What are the four types of bonds?
- The S&P 500 is at risk of developing a bearish head-and-shoulders top, Bank of America said.
- The S&P 500 could fall as much as 15% to 3800 if the bearish pattern successfully develops.
- The bond market and weakening financial conditions are warning of more stock market downside.
What are the three types of bonding?
as benchmark bond yields turn up with the promise of a Federal Reserve that is closing the purse-strings of too-loose monetary policy. Sign up for our Market Watch Newsletters here Economists and market participants are predicting three, four, maybe as ...
What is the weakest type of Bond?
What Is The Weakest Type Of Bond
- A. Hydrogen bond.
- B. Covalent bond.
- C. lonic bond.
- D. Metallic bond.
- Solution. (a) H-bond is only an interaction between electronegative element and hydrogen therefore, it is the weakest bond among all.
What are the 5 types of chemical bonds?
What are the 5 types of bonding? Types of chemical bonding. Atoms, ions, molecules. Gases, liquids and solids consist of atoms, ions and molecules. Covalent bond. With this bonding, atoms join into a molecule by sharing the electrons of their outer orbits. Ionic bond. Metal bond. Molecular bond, or Van der Vals bond.
What are the main types of bonding?
There are three primary types of bonding: ionic, covalent, and metallic.Ionic bonding.Covalent bonding.Metallic bonding.
What are the 4 types of bonds in chemistry with examples?
Different Types of Chemical Bonds with ExamplesIonic Bond. As the name suggests, ionic bonds are a result of the attraction between ions. ... Covalent Bond. In the case of a covalent bond, an atom shares one or more pairs of electrons with another atom and forms a bond. ... Hydrogen Bond. ... Metallic Bonds.
What are the 4 types of bonding that are important in the formation of minerals?
Chemical bonds in minerals are of four types: covalent, ionic, metallic, or Van der Waals, with covalent and ionic bonds most common. Two or more of these bond types can and do coexist in most minerals. Covalent bonds are very strong bonds formed when atoms share electrons with neighboring atoms.
What are the 5 chemical bonds?
Types of Chemical BondsIonic Bonds.Covalent Bonds.Hydrogen Bonds.Polar Bonds.
What are the 7 types of bonds?
Treasury bonds, GSE bonds, investment-grade bonds, high-yield bonds, foreign bonds, mortgage-backed bonds and municipal bonds - explained by Beth Stanton.
Why does each carbon bond to 4 other atoms?
Because carbon has four valence electrons and needs eight to satisfy the Octet rule, it can bond with up to four additional atoms, creating countless compound possibilities.
What is chemical bonding and its types?
Chemical bond is a bond by which the atoms in the molecule of a compound are combined and held together by a strong combining force. There are two types of chemical bonds; ionic bond and covalent bond.
What is physical bonding?
Bond means force of attraction. Matter is made up of molecules and and atoms. The physical force of attraction which holds atoms and molecules in a matter is called physical bond.
Which compound has all three types of bond?
Ammonium chlorideAmmonium chloride has all the three bonds present in it.
How many bond types are there?
The Bonds can be categorised into four variants: Corporate Bonds, Municipal Bonds, Government Bonds and Agency Bonds.
How many bonds are there in chemistry?
There are four types of bonds or interactions: ionic, covalent, hydrogen bonds, and van der Waals interactions.
What are ionic and covalent bonds?
There are primarily two forms of bonding that an atom can participate in: Covalent and Ionic. Covalent bonding involves the sharing of electrons between two or more atoms. Ionic bonds form when two or more ions come together and are held together by charge differences.
What are the four types of chemical bonds?
4 types of Chemical Bonds in Biology. There are four types of chemical bonds essential for life to exist: Ionic Bonds, Covalent Bonds, Hydrogen Bonds, and van der Waals interactions. We need all of these different kinds of bonds to play various roles in biochemical interactions. These bonds vary in their strengths.
What type of bond is bonded by electrons?
Covalent Bonds: atoms bonded by sharing electrons. Hydrogen Bonds: hydrogen attracts and bonds to neighboring negative charges. van der Waals interactions: intermolecular interactions that do not involve covalent bonds or ions.
What are the weakest bonds in chemistry?
Also note that in Chemistry, the weakest bonds are more commonly referred to as “dispersion forces .”. There are four types of chemical bonds essential for life to exist. Ionic Bonds: bonds formed between ions with opposite charges. Covalent Bonds: atoms bonded by sharing electrons.
Which bonds are strongest to weakest in biochemistry?
This means Ionic bonds tend to dissociate in water. Thus, we will think of these bonds in the following order (strongest to weakest): Covalent, Ionic, Hydrogen , and van der Waals.
How many types of bonds are there in carbon?
Four Types of Bonds. Now that we know the shape of carbon (a tetrahedron) as well as the three broad types of covalent bonds, we can better understand the four types of bonds a carbon molecule can form. As we work through each type, check to make sure the total bonds present in a carbon molecule equals to eight valence electrons.
What type of bond is type 3?
Type 3: Two Double Bonds. Diagram 4 shows an example of this carbon bond type. Even more complex than type two and type one, two double bonds are available for the bonding of an atom to carbon. One double bond means that four valence electrons from carbon are covalently shared with another atom. With two double bonds present, carbon's need of having eight valence electrons in its outer shell is fulfilled.
What are the bonds that carbon can form?
A fundamental element, important for living, there are four different types of bonds carbon can form. The bonds formed are called covalent bonds, which are created when two atoms share an electron (i.e. valence electrons) with each other.
How many valence electrons are needed for a covalent bond?
Eight valence electrons need to be present in carbon's outer shell, when considering covalent bonding. The three major types of covalent bonds are single, double, and triple bonds. A carbon atom can form the following bonds: Four single bonds. One double and two single bonds.
How many bonds are there in a carbon molecule?
Type 1: Four Single Bonds. Shown in diagram 2, think of this bond type as your simplest of them all. It is the basic tetrahedron shape of a carbon molecule. There are four single bonds available for the bonding to four atoms.
What type of bond is the last type of covalent bond?
This leads us to the last type of bond, a triple bond . A triple bond is a type of covalent bond where six electrons are shared between two atoms. The take away with each of these bonds is the word 'sharing.'. Remember this is the basis for covalent bonding.
How many electrons are in a single bond?
Single bonds are a type of covalent bond formed from the sharing of two electrons between two atoms, one electron from each atom. Now this is quite different from double bonds. Double bonds are a type of covalent bond where four electrons are shared between two atoms. This leads us to the last type of bond, a triple bond.
