The strong acids are hydrochloric acid Hydrochloric acid or muriatic acid is a colorless inorganic chemical system with the formula H₂O:HCl. Hydrochloric acid has a distinctive pungent smell. It is classified as strongly acidic and can attack the skin over a wide composition range, since the hydrogen chloride completely di… Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at 124.3 °C and contains 47.6% HBr by mass, which is 8.89 mol/L. Hydrobromic acid has a pKₐ of −9, makin… Perchloric acid is a mineral acid with the formula HClO₄. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid and nitric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperatu…Hydrochloric acid
Hydrobromic acid
Perchloric acid
What are the 7 strong acids?
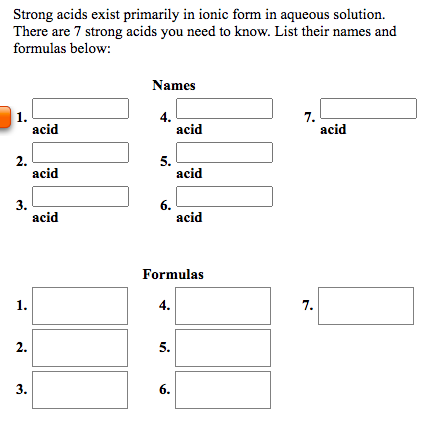
There are only a few (7) strong acids, so many people choose to memorize them. All the other acids are weak. The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid.
What is the formula for a strong acid?
The strength of an acid is determined by the H + ion concentration in its aqueous solution. For knowing whether an acid is a strong acid or not, look out the basic definition of strong acid Strong acid: A strong acid is a compound that is 100% ionized or completely dissociates in an aqueous solution and gives a high amount of hydrogen ions.
Is Koh a strong acid?
Ques-is koh a strong acid. Heres your answer!! KOH is potassium hydroxide. Since it is composed of the hydroxide anion (OH-), it is a strong base. In solution, the hydroxide anion will completely react with any available protons, that is why KOH is a strong base. It is not an acid of any type, weak or strong, since KOH does not contribute any protons to solution.
What are strong acids and bases?
Evaluate solution pH and pOH of strong acids or bases. Acids and bases that are completely ionized when dissolved in water are called strong acids and strong bases There are only a few strong acids and bases, and everyone should know their names and properties. These acids are often used in industry and everyday life.
What are the five strongest acids and bases?
Strong AcidsStrong AcidsStrong Baseshydrochloric acid (HCl)sodium hydroxide (NaOH)hydrobromic acid (HBr)potassium hydroxide (KOH)hydroiodic acid (Hl)calcium hydroxide (Ca(OH)2)nitric acid (HNO3)strontium hydroxide (Sr(OH)2)2 more rows•Feb 2, 2022
What are 5 weak acids?
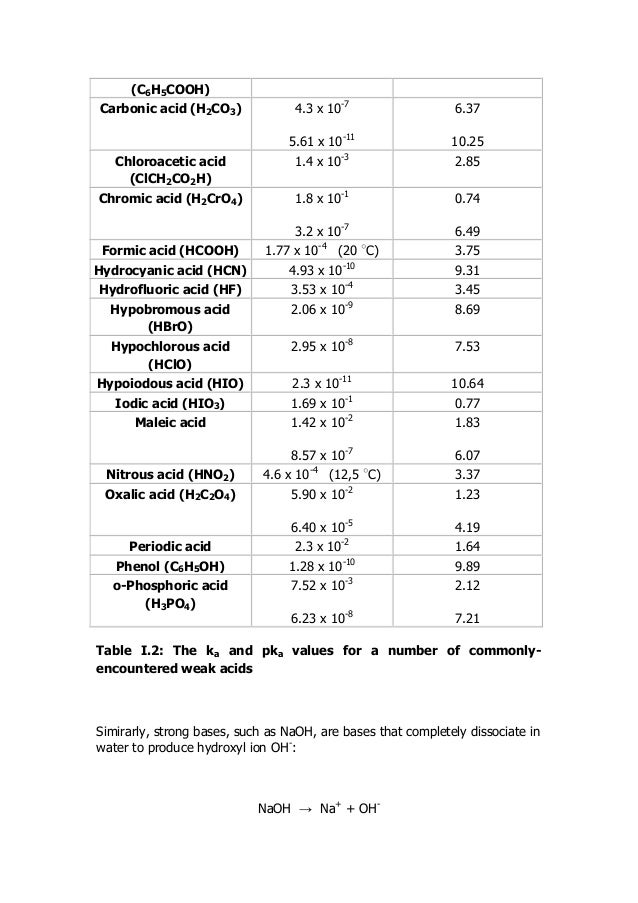
Examples of Weak AcidsFormic acid (chemical formula: HCOOH)Acetic acid (chemical formula: CH3COOH)Benzoic acid (chemical formula: C6H5COOH)Oxalic acid (chemical formula: C2H2O4)Hydrofluoric acid (chemical formula: HF)Nitrous acid (chemical formula: HNO2)Sulfurous acid (chemical formula: H2SO3)More items...
What are the examples of strong acids?
Hydrochloric Acid (HCl) Hydrochloric acid, also known as muriatic acid, is a chemical compound with the formula HCl. ... Hydrobromic Acid (HBr) ... Hydroiodic Acid (HI) ... Sulphuric Acid (H2SO4) ... Nitric Acid (HNO3) ... Perchloric Acid (HClO4) ... Chloric Acid (HClO3)
What are the big 6 strong acids?
They are H2SO4 (or sulfuric acid), HI (hydrologic acid), HBr (hydrobromic acid), HNO3 (nitric acid), HCl (hydrochloric acid) and HClO4 (perchloric acid). The mnemonic that I can use to help you memorize these six strong acids is: So I Brought No Clean Clothes. You have SO for sulfuric acid.
What are the 7 strong acids?
Strong acids consist of seven main acids – chloric acid, hydrobromic acid, hydrochloric acid, hydroiodic acid, nitric acid, perchloric acid, and sulfuric acid.
What are the 7 strong bases?
List of Strong Bases (8):LiOH (lithium hydroxide)NaOH (sodium hydroxide)KOH (potassium hydroxide)Ca(OH)2 (calcium hydroxide)RbOH (rubidium hydroxide)Sr(OH)2 (strontium hydroxide)CsOH (cesium hydroxide)Ba(OH)2 (barium hydroxide)
Which is most strong acid?
fluoroantimonic acidThe world's strongest superacid is fluoroantimonic acid, HSbF6. It is formed by mixing hydrogen fluoride (HF) and antimony pentafluoride (SbF5). Various mixtures produce the superacid, but mixing equal ratios of the two acids produces the strongest superacid known to man.
Which is strongest acid?
Fluoroantimonic acid is the strongest super-acid known in existence which is 100,000 billion billion billion times more acid than gastric acid (pH of -31.3.).
How do you remember the 7 strong acids?
0:002:05Easily Memorize 7 strong acids - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd then three on the chlorine. So chloric acid perchloric acid and hydrochloric acid hydrobromicMoreAnd then three on the chlorine. So chloric acid perchloric acid and hydrochloric acid hydrobromic acid and hydro attic acid. So it makes like it makes this almost like a seven.
Which acid is called the King of acids?
oxyacid: Sulfuric acid. Sulfuric acid is sometimes referred to as the “king of chemicals” because it is produced worldwide in... Due to its affinity for water, pure anhydrous sulfuric acid does not exist in nature.
Is NaOH a strong acid?
NaOH is a strong base as it completely dissolves in water to release hydroxide ions (along with sodium ions) which are responsible for the basic nature of an aqueous solution. A Compound that releases OH- ions in an aqueous solution is basic in nature. The pH value of NaOH is around 12 classifying it as a strong base.
What are the 6 strong acids and bases?
1 Answer. Oscar L. Acids: HCl , HBr , HI , HClO4 , HNO3 , H2SO4 . Bases: LiOH , NaOH , KOH , RbOH , CsOH , Ba(OH)2 .
What are the weakest acids?
Strong acids are 100% ionized in solution. Weak acids are only slightly ionized. Phosphoric acid is stronger than acetic acid and so is ionized to a greater extent. Acetic acid is stronger than carbonic acid, and so on....Strong and Weak Acids and Acid Ionization Constant.AcidConjugate BaseHCN (hydrocyanic acid) (weakest)CN− (cyanide ion) (strongest)8 more rows•May 14, 2019
What are the 7 weak bases?
Many weak bases are simply slightly soluble hydroxides, like magnesium hydroxide, while others are organic compounds.Ammonium Hydroxide (NH4OH)Aniline (C6H5NH2)Ammonia (NH3)Methylamine (CH3NH2)Ethylamine (CH3CH2NH2)Aluminum hydroxide (Al(OH)3)Magnesium Hydroxide (Mg(OH)2)Pyridine (C5H5N)More items...•
What are weak acids and its examples?
Examples of Weak AcidsCommon Weak Acidsacetic acid (ethanoic acid)CH3COOHformic acidHCOOHhydrocyanic acidHCNhydrofluoric acidHF4 more rows•Jan 29, 2020
What are two weak acids?
Weak Acids & BasesCommon Weak AcidsCommon Weak BasesFormicHCOOHammoniaAceticCH3COOHtrimethyl ammoniaTrichloroaceticCCl3COOHpyridineHydrofluoricHFammonium hydroxide5 more rows•Aug 15, 2020
What is a strong acid?
A strong acid is one which completely dissociates in its solvent. Under most definitions, the acid dissociates into a positively-charged hydrogen ion (proton) and a negatively-charged anion.
What makes an acid strong?
What makes them "strong" is the fact that they completely dissociate into their ions (H + and an anion) when they are mixed with water. Every other acid is a weak acid. Because there are only seven strong acids, it is easy to commit the list to memory. Note that some chemistry instructors may refer only to six strong acids.
What is the name of the acid that is colorless and has a pungent odor?
Hydrochloric acid: Hydrochloric acid also goes by the name of muriatic acid. The acid is colorless and has a pungent odor. Humans and most other animals secrete hydrochloric acid in the digestive system. The acid has many commercial applications. It is used to produce inorganic compounds, refine metals, pickle steel, and regulate pH. Of the common strong acids, it is one of the least hazardous to handle, least expensive, and easiest to store.
What is the purpose of nitric acid?
While colorless in pure form, nitric acid yellows over time as it decomposes into nitrogen oxides and water. In chemistry, one of its key uses is for nitration. This is where a nitro group gets added to a molecule (usually organic). Nitric acids finds use as an oxidant in nylon production, as the oxidizer in rocket fuel, and as an analytical reagent.
How much dissociation is a strong acid?
As the strong acids become more concentrated, they may be unable to fully dissociate. The rule of thumb is that a strong acid is 100 percent dissociated in solutions of 1.0 M or less. Cite this Article. Format.
Is S a solvent?
Here, S is a solvent molecule, such as water or dimethyl sulfoxide (DMSO).
Are Strong Acids Always Strong?
As the strong acids become more concentrated, they may be unable to fully dissociate. The rule of thumb is that a strong acid is 100 percent dissociated in solutions of 1.0 M or lower concentration .
What are strong acids?
The strong acids and bases are simply those that completely dissociate in water. Weak acids (which are all other acids) dissociate only a small amount.
What does it mean when an acid is strong?
When an acid is labelled as a strong acid, it actually has nothing to do with how powerful or corrosive it is . The “strength” of an acid simply refers to its ability to release hydrogen ions into a solution. Strong acids are acids that completely dissociate into their ions in water. This means that, in a solution, all of their molecules break up.
How many acids are there in chemistry?
If you’re taking chemistry, you’ll undoubtedly need to know the 7 strong acids. Read this guide to learn what the 7 strong acids are, why they’re important, and why they’re not necessarily the most dangerous acids you’ll be working with in the lab.
How to tell if an acid is strong or weak?
However, if you forget if an acid is strong or weak, you can also look at its equilibrium constant/acid dissociation constant (K a ). Strong acids will have large values for Ka, while weak acids will have very small values for Ka.
How many hydrogen cations are in a strong acid?
Strong acids yield at least one hydrogen cation (H +) per molecule. Weak acids, on the other hand, will dissociate less than 1%, which means very few of their molecules will break up to release a hydrogen ion.
Is a strong or weak acid the same as a concentrated acid?
It’s important to realize that strong/weak acids are not the same as concentrated/diluted acids. These terms are frequently misused and incorrectly substituted for each other! The concentration of an acid refers to how much water or solvent is in it.
Is acid corrosive or corrosive?
Just because an acid is strong doesn’t mean that it’s corrosive. Corrosiveness refers to how much a substance damages a surface it touches. Living tissue (such as skin, eyes, etc.) is often used as a reference point since people want to know any potential risks of substances they’re working with.
Which is the strongest acid in the world?
The record-holder used to be fluorosulfuric acid (HFSO 3 ), but the carborane superacids are hundreds of times stronger than fluorosulfuric acid and over a million times stronger ...
What is corrosive acid?
Corrosiveness is related to the negatively-charged part of the acid. Hydrofluoric acid (HF), for example, is so corrosve it dissolves glass. The fluoride ion attacks the silicon atom in silica glass while the proton is interacting with oxygen.
Is a strong acid a weak acid?
The good news, regarding the strong acid s, is any other acid is a weak acid. The 'strong acids' dissociate completely in water.
Is hydrofluoric acid corrosive?
Even though it is highly corrosive, hydrofluoric acid is not considered to be a strong acid because it does not completely dissociate in water. Helmenstine, Anne Marie, Ph.D. "Strong Acids and the World's Strongest Acid.".
How many strong acids are there in water?
Strong acids dissociate completely into their ions in water, yielding one or more protons (hydrogen cations ) per molecule. There are only 7 common strong acids .
What are the weak acids?
The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. The only weak acid formed by the reaction between hydrogen and a halogen is hydrofluoric acid (HF).
How much of ethanoic acid is converted to ions?
Only about 1% of ethanoic acid converts to ions, while the remainder is ethanoic acid. The reaction proceeds in both directions. The back reaction is more favorable than the forward reaction, so ions readily change back to weak acid and water.
How to tell if an acid is strong or weak?
Distinguishing Between Strong and Weak Acids. You can use the acid equilibrium constant K a or pK a to determine whether an acid is strong or weak. Strong acids have high K a or small pK a values, weak acids have very small K a values or large pK a values. Strong and Weak Vs. Concentrated and Dilute.
Which arrow points to the right of the reaction arrow?
Note the production of positively charged hydrogen ions and also the reaction arrow, which only points to the right. All of the reactant (acid) is ionized into product.
Is HCl a strong or weak acid?
Be careful not to confuse the terms strong and weak with concentrated and dilute. A concentrated acid is one that contains a low amount of water. In other words, the acid is concentrated. A dilute acid is an acidic solution that contains a lot of solvent. If you have 12 M acetic acid, it's concentrated, yet still a weak acid. No matter how much water you remove, that will be true. On the flip side, a 0.0005 M HCl solution is dilute, yet still strong.
Is 0.0005 M HCl strong?
On the flip side, a 0.0005 M HCl solution is dilute, yet still strong. Strong Vs. Corrosive. You can drink diluted acetic acid (the acid found in vinegar), yet drinking the same concentration of sulfuric acid would give you a chemical burn.
Which acid is considered a strong acid?
The following acids dissociate almost completely in water, so they are often considered to be strong acids, although they are not more acidic than the hydronium ion, H 3 O +: Some chemists consider the hydronium ion, bromic acid, periodic acid, perbromic acid, and periodic acid to be strong acids.
What is a strong acid?
Updated November 07, 2019. A strong acid is one that is completely dissociated or ionized in an aqueous solution. It is a chemical species with a high capacity to lose a proton, H +. In water, a strong acid loses one proton, which is captured by water to form the hydronium ion:
How many proton loss in a strong acid?
Diprotic and polyprotic acids may lose more than one proton, but the "strong acid" pKa value and reaction refer only to the loss of the first proton.
What is a superacid?
These are the "superacids," which are defined as acids that are more acidic than 100% sulfuric acid. The superacids permanently protonate water.
When an acid dissociates, what is the equilibrium?
Equilibrium: When an acid dissociates, equilibrium is reached with its conjugate base. In the case of strong acids, the equilibrium strongly favors the product or is to the right of a chemical equation. The conjugate base of a strong acid is much weaker than water as a base.
Is hydronium ion a strong acid?
Some chemists consider the hydronium ion, bromic acid, periodic acid, perbromic acid, and periodic acid to be strong acids.
Is hydrofluoric acid corrosive?
Most strong acids are corrosive, but some of the superacids are not. In contrast, some of the weak acids (e. g., hydrofluoric acid) can be highly corrosive. As acid concentration increases, the ability to dissociate diminishes.
How many words are in the list of strong acids?
In the list of strong acids, there are three h words, each starting with the prefix hydro. Chunk those three words together and learn the phrase “before in class ” to help remember them. This phrase is based on the letter immediately following the hydro- in the remaining strong acids.
Why are strong acids not named as such?
Strong acids are not named as such because they are more powerful than other acids. A strong acid is one that dissolves in water. The term strong in the name refers to the acid’s ability to release hydrogen (H +) molecules, which allows it to become ionized when placed into a solution of water. Weak acids do not have this ability.
What is sulfuric acid?
sulfuric acid - drain cleaner ingredient, wastewater treatment, steel manufacturing. Each of these acids can be used in many ways beyond the examples provided above. Most of their usage applications are specific to industrial and scientific laboratory settings.
How many strong bases are there?
Strong bases release hydroxide (OH-) ions and absorb hydrogen (H+) molecules. There are eight strong bases.
What do you learn in chemistry?
Studying chemistry involves learning about acids and bases. If you’re taking a chemistry class, you’ll probably need to memorize the strong acids and bases. Use this list of strong acids and bases as a tool to help you learn the names and chemical composition of each one.
What are the acids in vinegar?
Acids Found at Home 1 Acetic acid (HC2H3O2) is found in vinegar as well as products that contain vinegar, such as ketchup. 2 Citric acid (H3C6H5O7) is found in citrus fruits. It is also used in jams and jellies and to add a tangy flavor to other foods. 3 Lactic acid (C3H6O3) is found in milk and other dairy products. 4 Ascorbic acid (C6H8O6) is vitamin C. It is found in citrus fruits as well as some other fruits and juices. 5 Sulfuric acid (H2SO4) is found in car batteries and some drain cleaners.
Where can sulfuric acid be found?
It is found in citrus fruits as well as some other fruits and juices. Sulfuric acid (H2SO4) is found in car batteries and some drain cleaners. Helmenstine, Anne Marie, Ph.D. "5 Common Acids in the Home.".
What is the strength of an acid?
Strong acids are molecules that completely dissociate into their ions when it is in water. In other words, acids release H + ions into the solution by their complete ionization. The strength of an acid is characterized by their acid dissociation constant values (K a ). Normally, strong acids have a very large K a value.
What is the difference between strong and weak acids?
The main difference between strong and weak acids is that strong acids dissociate completely in aqueous solutions whereas weak acids partially dissociate in aqueous solutions.
What is weak acid?
Weak acids are molecules that partially dissociate into ions in aqueous solutions. Weak acids do not release all the H + ions to the solution. The acid dissociation constant (K a) is a small value than that of strong acids. The pH of the solution is about 3-5.
How are acids determined?
The strength of an acid is determined by the polarity and the atomic sizes of the acid molecule. According to the way that acid molecules dissociate in water, there are two types of acids as strong acids and weak acids. The main difference between strong and weak acids is that strong acids dissociate completely in aqueous solutions whereas weak acids partially dissociate in aqueous solutions.
Why do acids lose protons?
These protons are easily released due to the high polarity of the bond between H atom and the rest of the molecule. This polarity is determined by the electronegativity of two atoms involved in this bond. The deprotonation (removal of a proton) of a strong acid depends on the polarity and the size of the anion which the proton is attached to.
Why is pH influenced by acid?
On the other hand, the pH of the solution is greatly influenced by strong acids because strong acids release H+ ions to the solution. The pH depends on the H+ concentration. The relationship between H+ concentration and the pH can be given as below. If the acid is a strong acid, the pH value is a very small value.
Which constant is higher for strong acids?
Strong Acid: The acid dissociation constant K a is a higher value for strong acids.
