The following points highlight the top ten properties of water molecules. The properties are: 1. Temperature and Physical State 2. Absorption and Dissipation of Heat 3. Melting and Vaporizing Water 4.
What are the 4 main characteristics of water?
- Turbidity of Water.
- Colour.
- Taste and Odour.
- Temperature of Water.
- Specific Conductivity.
- Total Solids and Suspended Solids.
- pH value of Water.
- Hardness of Water.
Does water have atoms or molecules?
Two Hydrogen atoms and one Oxygen atom combine into a water molecule. Water is made up of two important elements, Hydrogen and Oxygen. It's chemical formula is H2O. If you are talking about only one molecule, then this picture can help you understand better. So, by this we understand that There are two atoms of hydrogen in one molecule of water.
What are the physical and chemical characteristics of water?
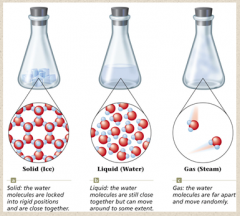
PHYSICAL AND CHEMICAL PROPERTIES OF WATER Water is a chemical substance with the chemical formula H2O. Its molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state, water vapor or steam.
What is water's unique characteristics?
5 Unique Properties Of Water High Polarity Lower Density Of Ice The reason icebergs are floating on the sea surface is because of this lower density. ... High Heat Of Evaporation When water starts evaporating off of a surface, it creates an effect of cooling. ... More items...

What are the 5 main characteristics of water?
5 Physical Characteristics of Water | Water ManagementSuspended Solids: Suspended solids in water may consist of inorganic or organic particles or of immiscible liquids (oils or greases). ... Turbidity: ... Colour: ... Taste and Odour: ... Temperature:
What characteristics of water molecules make it unique?
Water is made up of two hydrogen (H) atoms and an oxygen (O) atom. It is unique in that it is bipolar, where the molecule has a slightly positive charge on one side (where hydrogen atoms are attached), and slightly negative on the other (just oxygen).
What are the properties of water molecules?
Water molecules are polar, so they form hydrogen bonds. This gives water unique properties, such as a relatively high boiling point, high specific heat, cohesion, adhesion and density.
What are the 4 characteristic of water?
These include: Cohesion, Adhesion, High surface tension, High specific heat, High Heat of vaporization, and the fact that ice floats (Ice is less dense as a solid than liquid water).
What are the 7 characteristics of water?
The main properties of water are its polarity, cohesion, adhesion, surface tension, high specific heat, and evaporative cooling. A water molecule is slightly charged on both ends. This is because oxygen is more electronegative than hydrogen.
What are the 6 main properties of water?
The properties of water include cohesion, adhesion, capillary action, surface tension, the ability to dissolve many substances, and high specific heat. The tendency for water molecules to form weak bonds and stick to each other is called cohesion.
What are the molecules of water?
A water molecule has three atoms: two hydrogen (H) atoms and one oxygen (O) atom. That's why water is sometimes referred to as H2O. A single drop of water contains billions of water molecules.
What are two characteristics or properties of the water molecule due to its polarity?
The polarity of water molecules means that molecules of water will stick to each other. This is called hydrogen bonding. Polarity makes water a good solvent, gives it the ability to stick to itself (cohesion), stick to other substances (adhesion), and have surface tension (due to hydrogen bonding).
What characteristic of water molecules account for its polarity?
Polarity: Although the net charge of a water molecule is zero, water is polar because of its shape. The hydrogen ends of the molecule are positive and the oxygen end is negative. This causes water molecules to attract each other and other polar molecules.
What are the 3 characteristics of water?
Physical characteristics of water qualityColor – pure water is colorless; colored water can indicate pollution. ... Turbidity – pure water is clear and does not absorb light. ... Taste and odor – pure water is always tasteless and odorless.More items...
How many characteristics of water are there?
Physical characteristics of water (temperature, colour, taste, odour and etc.) are determined by senses of touch, sight, smell and taste. For example temperature by touch, colour, floating debris, turbidity and suspended solids by sight, and taste and odour by smell.
What are the main quality characteristics of water?
However, there are eight key important characteristics of a water quality measurement: temperature, clarity, conductivity, pH, alkalinity, chlorine, hardness, and dissolved oxygen.
What makes water such a unique characteristic compared to other liquid solvents?
Water as the "Universal Solvent" Because of water's polarity, it is able to dissolve or dissociate many particles. Oxygen has a slightly negative charge, while the two hydrogens have a slightly positive charge.
Which of the following are among the unique characteristics of water quizlet?
Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions that leads to the generation of pH.
What is hydrogen bond How can it explain the various unique properties of water?
This sticking together of like substances is called cohesion. Depending on how attracted molecules of the same substance are to one another, the substance will be more or less cohesive. Hydrogen bonds cause water to be exceptionally attracted to each other. Therefore, water is very cohesive.
What is the unique defining property of atoms of a particular element?
Subatomic particles of use in biology. The atomic number is the number of protons an atom has. It is characteristic and unique for each element. The atomic mass (also referred to as the atomic weight) is the number of protons and neutrons in an atom.
What are the characteristics of water?
Characteristics of Water – Physical, Chemical and Biological. Water has three characteristics, i.e. physical, chemical and biological characteristics. The raw treated water can be checked and analysed by studying and testing these characteristics as explained below: 1. Turbidity of Water.
What is the unit of color in water?
2. Colour. The presence of colour in water is not objectionable from health point of view, but may spoil the colour of the clothes being washed. The standard unit of colour is that which is produced by one milligram of platinum cobalt dissolved in one litre of distilled water.
How to measure turbidity?
The turbidity is measured by a turbidity rod or by a turbidity meter with optical observations and is expressed as the amount of suspended matter in mg/l or parts per million (ppm). For water, ppm and mg/l are approximately equal. The standard unit is that which is produced by one milligram of finely divided silica (fuller’s earth) in one litre of distilled water.
How to measure the extent of taste in water?
The extent of taste or odour present in a particular sample of water is measured by a term called odour intensity, which is related with the threshold odour or threshold odour number. Water to be tested is therefore gradually diluted with odour free water, and the mixture at which the detection of odour by human observation is just lost, is determined. The number of times the sample is diluted represents the threshold odour number. For public supplies, the water should generally free from odour, i.e. the threshold number should be 1 and should never exceed 3.
How to find suspended solids in water?
The suspended solid can be found by filtering the water sample. Total permissible amount of solids in water is generally limited to 500 ppm.
What is the pH of water?
2. pH value of Water. If concentration increases, pH decreases and then it will be acidic. If concentration decreases, pH increases and then it will be alkaline. pH + pOH = 14 if the pH of water is more than 7, it will be alkaline and if it is less than 7, it will be acidic.
What temperature should water be?
For potable water, temperature of about about C is desirable. It should not be more than C.
What is the measure of water's ability to attract other types of molecules?
Adhesiveness is a measure of water's ability to attract other types of molecules. Water is adhesive to molecules capable of forming hydrogen bonds with it. Adhesion and cohesion lead to capillary action, which is seen when the water rises up a narrow glass tube or within the stems of plants.
What Is Water?
Water is a chemical compound. Each molecule of water, H 2 O or HOH, consists of two atoms of hydrogen bonded to one atom of oxygen .
Why is water a cohesive substance?
Cohesion is a key property of water. Because of the polarity of the molecules, water molecules are attracted to each other. Hydrogen bonds form between neighboring molecules. Because of its cohesiveness, water remains a liquid at normal temperatures rather than vaporizing into a gas. Cohesiveness also leads to high surface tension.
What are the other names for water?
Other names for water are dihydrogen monoxide, oxidane, hydroxylic acid, and hydrogen hydroxide.
What is the property of water that leads to high surface tension?
Cohesiveness also leads to high surface tension. An example of the surface tension is seen by beading of water on surfaces and by the ability of insects to walk on liquid water without sinking. Adhesion is another property of water. Adhesiveness is a measure of water's ability to attract other types of molecules.
Why does water have a lower density?
Hydrogen bonds between water molecules are responsible for the lower density of ice. An important consequence is that lakes and rivers freeze from the top down, with ice floating on water. Pure liquid water at room temperature is odorless, tasteless, and nearly colorless.
Which compound is bent in the gas phase?
Each molecule is bent, with the negatively charged oxygen on one side and the pair of positive-charged hydrogen molecules on the other side of the molecule. Water is the only common compound that exists in solid, liquid, and gas phase under ordinary, natural conditions.
What is the chemical formula for water?
Water is a chemical compound and polar molecule, which is liquid at standard temperature and pressure. It has the chemical formula H 2 O , meaning that one molecule of water is composed of two hydrogen atoms and one oxygen atom. Water is found almost everywhere on earth ...
Why is water a solvent?
The solvent properties of water are vital in biology, because many biochemical reactions take place only within aqueous solutions (e.g., reactions in the cytoplasm and blood). In addition, water is used to transport biological molecules. When an ionic or polar compound enters water, it is surrounded by water molecules.
Why is hydrogen bonding important?
The strong hydrogen bonds give water a high cohesiveness and, consequently, surface tension. This is evident when small quantities of water are put onto a nonsoluble surface and the water stays together as drops. This feature is important when water is carried through xylem up stems in plants; the strong intermolecular attractions hold the water column together, and prevent tension caused by transpiration pull. Other liquids with lower surface tension would have a higher tendency to "rip", forming vacuum or air pockets and rendering the xylem vessel inoperative.
Why does water have a high boiling point?
This relatively weak (relative to the covalent bonds within the water molecule itself) attraction results in physical properties such as a relatively high boiling point, because a lot of heat energy is necessary to break the hydrogen bonds between molecules. For example, sulfur is the element below oxygen ...
What is a molecule with a charge difference called?
A molecule with such a charge difference is called a dipole. The charge differences cause water molecules to be attracted to each other (the relatively positive areas being attracted to the relatively negative areas) and to other polar molecules. This attraction is known as hydrogen bonding.
How does water split?
Electrolysis. Water can be split into its constituent elements, hydrogen and oxygen, by passing a current through it. This process is called electrolysis. Water molecules naturally disassociate into H + and OH - ions, which are pulled toward the cathode and anode, respectively.
What is the liquid water path?
The liquid water path is a measure of the amount of liquid water in an air column.
What Are The Properties of Water?
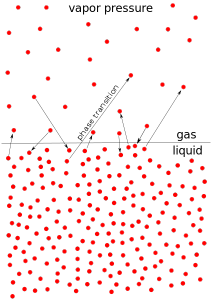
When they are frozen as ice, a definite pattern exists. In liquid form, the pattern is altered, as ice melts, hydrogen bonds are broken and water increases in density up to a temperature of 4°C as the pattern collapses and becomes denser. When the temperature increases from 4°C more bonds are broken because of thermal agitation, and water becomes less dense. At 100°C hydrogen bonds may break completely, resulting in escape of water molecules as vapor.
What is the structure of water?
Structure of water molecule is made up of one molecule of oxygen and two molecules of hydrogen bonded covalently. Water (H 2 O) essentially considered one of the most important substances found on the earth. It covers over 70% of the earth’s surface and makes up as much as 95% of the living organisms. It is virtually unique among liquids ...
How does water adhesion work?
Adhesion of Water. The adhesion of water is explained by hydrogen bonding of water molecules to other polar surfaces. The strong dipole of water exerts electrostatic and gravitational forces on charged electrovalent compounds and on the dipoles of polar, covalent compounds.
What happens when hydrogen bonds break?
At 100°C hydrogen bonds may break completely, resulting in escape of water molecules as vapor. The maximum density of water occurs at 4°C rather than at a minimum temperature like most substances has a profound effect on aquatic life.
Why is water's heat of vaporization so high?
Perhaps the most important consequent of water’s high heat of vaporization is the cooling effect as water evaporates from living surface.
Why is water polarized?
Because of this asymmetry of hydrogen atoms water has a strong dipole movement, i.e., it is. highly polarized with a strong separation of positive and negative charge. Because of this polarization, it readily shares its positively charged hydrogen with the negatively charged oxygen of other molecules.
Why is oxygen unique among liquids?
It covers over 70% of the earth’s surface and makes up as much as 95% of the living organisms. It is virtually unique among liquids because of molecular structure. As a result of the strong attraction of the oxygen nucleus for the electrons of hydrogen the hydrogen atoms are distorted from their usual position.
How many electrons does a water molecule have?
water molecule. A water molecule is made up of two hydrogen atoms and one oxygen atom. A single oxygen atom contains six electrons in its outer shell, which can hold a total of eight electrons. When two hydrogen atoms are bound to an oxygen atom, the outer electron shell of oxygen is filled. Encyclopædia Britannica, Inc.
Why does water appear blue?
In small quantities water appears colourless, but water actually has an intrinsic blue colour caused by the slight absorption of light at red wavelengths. For larger bodies of water—ponds, rivers, lakes, and oceans—water appears blue on clear days because it mirrors the blueness of the sky. On overcast days, larger water bodies appear gray.
How many neutrons are in water?
The water molecule is composed of two hydrogen atoms, each linked by a single chemical bond to an oxygen atom. Most hydrogen atoms have a nucleus consisting solely of a proton. Two isotopic forms, deuterium and tritium, in which the atomic nuclei also contain one and two neutrons, respectively, are found to a small degree in water. Deuterium oxide (D 2 O), called heavy water, is important in chemical research and is also used as a neutron moderator in some nuclear reactors.
Why does a cold water bottle make you sweat?
A cold water bottle appears to sweat because it’s a cooling source for the water vapour in the layer of air that surrounds the bottle. Air that is relatively warm can hold more water vapour than cooler air. When the cold water bottle is introduced, the warm air near the bottle cools and some of the water vapour condenses into liquid water, which is then deposited on the outside of the bottle.
What is H2O water?
Alternative Title: H2O. Water, a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid states. It is one of the most plentiful and essential of compounds.
Which air holds more water?
Air that is relatively warm can hold more water vapour than cooler air. When the cold water bottle is introduced, the warm air near the bottle cools and some of the water vapour condenses into liquid water, which is then deposited on the outside of the bottle.
What temperature does water boil?
At sea level, atmospheric pressure is high, and water boils at 100 °C (212 °F); at higher altitudes it is lower, so water boils at a lower temperature.
Why do water molecules stay close to each other?
Water molecules stay close to each other ( cohesion ), due to the collective action of hydrogen bonds between water molecules. These hydrogen bonds are constantly breaking, with new bonds being formed with different water molecules; but at any given time in a sample of liquid water, a large portion of the molecules are held together by such bonds.
How dense is water?
The density varies with temperature, but not linearly: as the temperature increases, the density rises to a peak at 3.98 °C (39.16 °F) and then decreases; this is unusual. Regular, hexagonal ice is also less dense than liquid water—upon freezing, the density of water decreases by about 9%.
How many polymorphs does water have?
Due to the existence of many polymorphs (forms) of ice, water has other triple points, which have either three polymorphs of ice or two polymorphs of ice and liquid in equilibrium. Gustav Heinrich Johann Apollon Tammann in Göttingen produced data on several other triple points in the early 20th century.
How does the density of saltwater affect the temperature of the water?
The density of saltwater depends on the dissolved salt content as well as the temperature. Ice still floats in the oceans, otherwise, they would freeze from the bottom up. However, the salt content of oceans lowers the freezing point by about 1.9 °C (see here for explanation) and lowers the temperature of the density maximum of water to the former freezing point at 0 °C. This is why, in ocean water, the downward convection of colder water is not blocked by an expansion of water as it becomes colder near the freezing point. The oceans' cold water near the freezing point continues to sink. So creatures that live at the bottom of cold oceans like the Arctic Ocean generally live in water 4 °C colder than at the bottom of frozen-over fresh water lakes and rivers.
What is the most common form of water?
Within the Earth's atmosphere and surface, the liquid phase is the most common and is the form that is generally denoted by the word "water". The solid phase of water is known as ice and commonly takes the structure of hard, amalgamated crystals, such as ice cubes, or loosely accumulated granular crystals, like snow.
Why does water act as a Lewis base?
Because the oxygen atom in water has two lone pairs, water often acts as a Lewis base, or electron-pair donor, in reactions with Lewis acids, although it can also react with Lewis bases, forming hydrogen bonds between the electron pair donors and the hydrogen atoms of water.
How hot is water?
Water has a very high specific heat capacity of 4184 J/ (kg·K) at 25 °C – the second-highest among all the heteroatomic species (after ammonia ), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive hydrogen bonding between its molecules. These two unusual properties allow water to moderate Earth's climate by buffering large fluctuations in temperature. Most of the additional energy stored in the climate system since 1970 has accumulated in the oceans.
How are water molecules connected?
The atoms in a water molecule are connected to each other by polar covalent bonds. The molecule in itself is electrically neutral but polar with negative and positive charges localized in different areas.
Why is the water molecule polar?
Thus, the structure of the water molecule is an angular of bent structure. The molecule of water is polar because oxygen is more electronegative than hydrogen.
How are water molecules held together?
In the liquid phase, water molecules are held together by intermolecular hydrogen bonds.
What is the solid form of water?
In solid phase. The solid form of water is ice, which can exist in different crystalline forms depending on the conditions for the freezing of water. In the regular hexagonal ice, each oxygen atom is tetrahedrally surrounded by four other oxygen atoms, whereas one hydrogen atom lies in between each pair of oxygen.
When the number of water molecules in a solution is more than the solute molecules, the interactions lead to the formation?
When the number of water molecules in a solution is more than the solute molecules, the interactions lead to the formation of a three-dimensional sphere of water , called the hydration shell around the solute molecules.
Why is water a good medium for dissolution?
Water has a high dipole moment which makes it an ideal medium for the dissolution of a wide variety of compounds. The high specific heat capacity of water enables it to absorb the heat of various biochemical and physiological reactions, going on inside the body, with the minimum rise of temperature.
What is the meaning of water?
Water is an inorganic liquid chemical that is colorless, odorless, tasteless that makes up most of the Earth’s hydrosphere and the fluids in the body of all living beings. Water is an extremely important component for the existence of life as it is vital for all biological processes. It doesn’t, however, have any calorific value ...
General
The Dipolar Nature of The Water Molecule
- An important feature of the water molecule is its polar nature. The water molecule forms an angle, with hydrogen atoms at the tips and oxygen at the vertex. Since oxygen has a higher electronegativity than hydrogen, the side of the molecule with the oxygen atom has a partial negative charge. A molecule with such a charge difference is called a dipo...
Structure of Water and Ice
- Shown above is a side by side comparison of a box 10 Angstroms across. It clearly shows that ice takes up more space because of the hydrogen bonding that occurs when the state changes from liquid to solid. In ice Ih, each water forms four hydrogen bonds with O---O distances of 2.76 Angstroms to the nearest oxygen neighbor. Because of ordered structure in ice there are less H2…
Water as A Solvent
- Water is also a good solvent due to its polarity. The solvent properties of water are vital in biology, because many biochemical reactions take place only within aqueous solutions (e.g., reactions in the cytoplasm and blood). In addition, water is used to transport biological molecules. When an ionic or polar compound enters water, it is surrounded by water molecules. The relatively small s…
Cohesion and Surface Tension
- The strong hydrogen bonds give water a high cohesiveness and, consequently, surface tension. This is evident when small quantities of water are put onto a nonsoluble surface and the water stays together as drops. This feature is important when water is carried through xylem up stems in plants; the strong intermolecular attractions hold the water column together, and prevent tens…
Conductivity
- Pure water is actually a good insulator (poor conductor), meaning that it does not conduct electricity well. Because water is such a good solvent, however, it often has some solute dissolved in it, most frequently salt. If water has such impurities, then it can conduct electricity much better, because impurities such as salt comprise free ions in aqueous solution by which an electric curr…
Electrolysis
- Water can be split into its constituent elements, hydrogen and oxygen, by passing a current through it. This process is called electrolysis. Water molecules naturally disassociate into H+ and OH- ions, which are pulled toward the cathode and anode, respectively. At the cathode, two H+ ions pick up electrons and form H2 gas. At the anode, four OH- ions combine and release O2gas…
Reactivity
- Chemically, water is amphoteric: able to act as an acid or base. Occasionally the term hydroxic acid is used when water acts as an acid in a chemical reaction. At a pH of 7 (neutral), the concentration of hydroxide ions (OH-) is equal to that of the hydronium (H3O+) or hydrogen ions (H+) ions. If the equilibrium is disturbed, the solution becomes acidic (higher concentration of hy…
Purifying Water
- Purified water is needed for many industrial applications, as well as for consumption. Humans require water that does not contain too much salt or other impurities. Common impurities include chemicals or harmful bacteria. Some solutes are acceptable and even desirable for perceived taste enhancement. Water that is suitable for drinking is termed potable water. Six popular meth…
Wasting Water
- Wasting wateris the abuse of water, i.e. using it unnecessarily. An example is the use of water, particularly water purified to human safe drinking standards, in unnecessary irrigation. Also, in homes, water may be wasted if the toilet is flushed unnecessarily or the tank leaks. Causing water to become polluted may be the biggest single abuse of water. To the extent that a pollutant limit…