Two other models proposed for the atom were the cubic model and the Saturnian model. In the cubic model, the electrons were imagined to lie at the corners of a cube. In the Saturnian model, the electrons were imagined to orbit a very big, heavy nucleus. Rutherford
Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson, OM, FRS, HFRSE, LLD, was a New Zealand physicist who came to be known as the father of nuclear physics. Encyclopædia Britannica considers him to be the greatest experimentalist since Michael Faraday.
- John Dalton's atomic model: Dalton´s Billiard Ball (Solid Sphere) Model.
- J.J. Thomson's model: Plum Pudding model.
- Ernest Rutherford's model: Nuclear model.
- Niels Bohr's model: Planetary model.
- Erwin Schrödinger's model: Electron Cloud Model/Quantum Model.
How could we build models of other atoms?
Use the number of protons, neutrons, and electrons to draw a model of the atom, identify the element, and determine the mass and charge. Predict how addition or subtraction of a proton, neutron, or electron will change the element, the charge, and the mass.
What is the best model of an atom?
The models differ in a few ways, but importantly: [8]
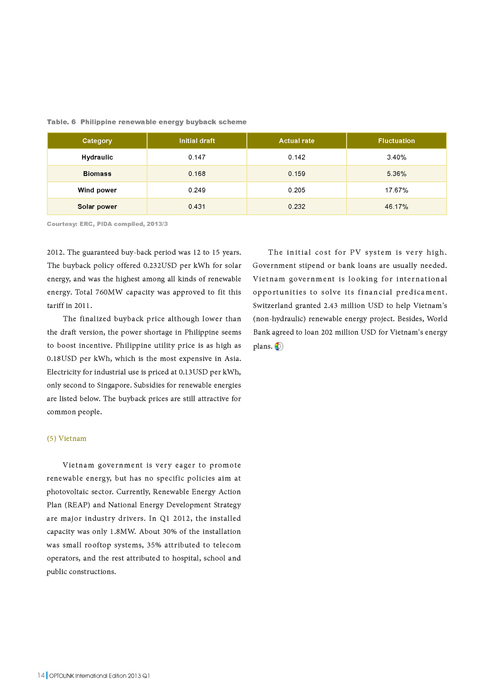
- The quantum model represents the true 3D space an atom exists in, the Bohr model only represents a 2D space.
- The Bohr model was a 1D model that used one quantum number to describe the distribution of electrons in the atom. ...
- The Bohr Model treats the electron as a particle in fixed orbits around the nucleus. ...
What are the different types of atomic models?
The five atomic models that shaped the modern atomic theory are:
- John Dalton’s atomic model
- J.J. ...
- Ernest Rutherford’s atomic model
- Niels Bohr’s atomic model
- Quantum Numbers/model I. ...
- All matter is made up of particles called atoms.
- Atoms are indivisible particles.
- Specific elements generally have only one type of atom present in them.
- Each atom has a constant mass respectively that differs from element to element.
How many different types of atomic models are there?
What are the different models of atoms?
- John Dalton's atomic model. Ilustration of Dalton's perception of atom.
- Plum pudding model.
- Rutherford's model of the atom.
- Bohr's model of the atom.
- Electron Cloud Model/Quantum Mechanics Model of Atom.
- Basic description of the quantum mechanical atomic model:
- Sources:

What are the 4 atomic models?
What Are the 4 Atomic Models?The Plum Pudding Model. The so-called plum pudding model was proposed by the scientist J.J. ... Planetary Model. This theory was proposed by the Nobel Prize winning chemist Ernest Rutherford in 1911 and is sometimes called the Rutherford model. ... Bohr Model. ... Electron Cloud Model.
What are the 3 atom models?
Developing models of atomsDalton's model (1803)Thomson's model (1897)The Geiger-Marsden experiment (1909 - 1911)Bohr's model (1913)
What are the 7 models of the atom?
Terms in this set (7)Democritus Model. Anything is made up of small things that look just like it. ... John Dalton Model. A tiny, hard, round, spire. ... JJ Tomson Model. Plum Puddling model, Not very dense. ... Rutherford Model. ... Neils Bohr Model. ... Louis de Broglie & Erwin Schrodinger Model. ... James Chadwick.
What are the 6 models of atom?
The models of an atom are:John Dalton's atomic model: Dalton's Billiard Ball (Solid Sphere) Model. ... J.J. Thomson's model: Plum Pudding model. ... Ernest Rutherford's model: Nuclear model. ... Niels Bohr's model: Planetary model. ... Erwin Schrodinger's model: Electron Cloud Model/Quantum Model. ... Wave mechanical model.
What are the 5 models of the atom?
Although the awareness of atom existence goes way back to the antique period of the world history (Greek conception of atom), this article will be mainly about five basic atomic models, from which each one has somehow contributed to how we percept the structure of atom itself - Dalton´s Billiard Ball Model, J.J ...
What is the first atom model?
In 1898, J. J. Thomson proposed the first of many atomic models to come. He proposed that an atom is shaped like a sphere with a radius of approximately 10-10m, where the positive charge is uniformly distributed. The electrons are embedded in this sphere so as to give the most stable electrostatic arrangement.
What are the atomic models in order?
A timeline of atomic modelsAtomic model (1808)Plum-pudding model (1904)Nuclear model (1911)Planetary model (1913)Quantum mechanical model (1926-present)
What is the latest model of atom?
Generally speaking, the Bohr model encapsulates the modern understanding of the atom. This model is often depicted in artwork showing a central atomic nucleus and oval lines representing the orbits of the electrons.
Who made the first atom model?
John Dalton was the first to adapt Democritus' theory into the first modern atomic model.
How do you identify the name of an atom model?
0:073:05Atom Identification : Chem Class - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo one way that we can identify our atoms is by looking at the nucleus. So if we know it our nucleusMoreSo one way that we can identify our atoms is by looking at the nucleus. So if we know it our nucleus looks like we can simply count the number of protons.
What are the 4 quantum numbers?
In atoms, there are a total of four quantum numbers: the principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron spin quantum number (ms).
What was Bohr's model of the atom called?
Bohr's work was primarily based on the emission spectra of hydrogen. This is also referred to as the planetary model of the atom. It explained the inner workings of the hydrogen atom. Bohr was awarded the Nobel Prize in physics in 1922 for his work.
What are the atomic models in order?
A timeline of atomic modelsAtomic model (1808)Plum-pudding model (1904)Nuclear model (1911)Planetary model (1913)Quantum mechanical model (1926-present)
Which atom model is used today?
electron cloudThe modern model of atomic structure is called the “electron cloud” model.
What was JJ Thomson atomic model?
In 1897, J. J. Thomson discovered the first subatomic particle, the electron, while researching cathode rays. To explain the neutrality of atoms, Thomson proposed a model of the atom in which negative electrons are scattered throughout a sphere of positive charge. He called his atom the plum pudding model.
What was Bohr's model of the atom called?
Bohr's work was primarily based on the emission spectra of hydrogen. This is also referred to as the planetary model of the atom. It explained the inner workings of the hydrogen atom. Bohr was awarded the Nobel Prize in physics in 1922 for his work.
What are the 4 atomic models?
Dalton’s Model of the Atom, J.J Thomson’s Model of Atom, Rutherford’s Atomic Model, and Neil Bohr’s Atomic Theory.
What is the most accurate atomic model?
Neil Bohr’s Atomic Model is the most accurate atomic model.
What was the first atomic model?
Dalton’s Model of the Atom was the first atomic model.
What is the current atomic model called?
The current model of the atom is called the quantum model of the atom.
Who is the father of atomic theory?
John Dalton is the father of atomic theory.
What are the drawbacks of Thomson’s model of the atom?
The reason for the failure of the plum pudding model are as follows- i. He could not offer any experimental support to his atomic model. ii. He cou...
What are the limitations of Rutherford’s model of the atom?
The drawback of the Rutherford model is: It couldn’t explain the stability of an atom. According to the model, the orbital revolution of electrons...
What are the five atomic models?
The five atom is models are as follows: John Dalton’s atomic model J.J Thomson’s atomic model- Plum pudding model Ernest Rutherford model- Nuclear...
What is the most accurate atomic model?
The most accurate atomic model was given by Neil Bohr and is called Bohr’s model of atom or Planetary model.
What are the postulates of Dalton’s Atomic Theory?
The main postulates of Dalton’s atomic theory are: - The matter is composed of a large number of extremely small particles called an atom. - Atoms...
Which model was the first atomic model?
Ans.3 Dalton’s Model of the Atom was the first atomic model.
Which model was not able to define the effect of magnetic field and electric field on the spectra of atom?
Bohr’s model was not able to define the effect of magnetic field and electric field on the spectra of atoms.
What is the smallest particle in an element?
An atom is the smallest particle of every element. The word atom came from the Greek word Atomos which means indivisible. Atoms are made of electrons, Protons, and Neutrons. Protons and Neutrons reside in the nucleus of the atom and electrons orbit the nucleus. Atoms always have an equal number of protons and electrons and the number of neutrons and protons is usually the same as well. Protons and Neutrons are held together in the nucleus by a strong force called Strong Nuclear force.
What is the center of an atom?
On the basis of this experiment, Rutherford stated that there is a positively charged spherical centre in an atom called the nucleus, and nearly all the mass of an atom (having a radius 10-10m) is packed in the nucleus (having a radius of 10–15 m).
What does Dalton say about atoms?
Dalton said that the atoms of the same element are similar in all respects and the atoms of different elements are different in all respects.
Why was Bohr's model adopted?
Later, because of a few issues like the stability of the nucleus Bo hr’s model was adopted.
Where do electrons revolve?
He stated that electrons revolve around the nucleus in a well-defined path called “orbit”.
What are the four atomic models?
In this article, we learned in detail the four Atomic models- John Dalton’s atomic model, J.J Thomson’s atomic model- Plum pudding model, Ernest Rutherford model- Nuclear model of an atom, Neil Bohr’s model of the atom- Planetary model. Also, we learned their limitations, postulates, etc.
How many subatomic particles are there in an atom?
Atomic Models: We very well know the fact that everything around us is made of matter. We also know that there are three subatomic particles- electrons, protons and neutrons. But where in an atom are all these subatomic particles located, and what is the structure of an atom? This article will get answers to all these questions and how various discoveries were made of Atomic Models.
What did the discovery of electrons prove?
The discovery of electrons proved for the first time that atoms themselves were made of fundamental particles, which were the constituents of all atoms and therefore, atoms did have a fine structure that was unique to every different atom.
Why is the orbital revolution of electrons around the nucleus not stable?
According to the model, the orbital revolution of electrons around the nucleus is not stable as it will undergo acceleration and emit energy which will result in the loss of energy of electrons, and they will ultimately collide with the nucleus.
Where do electrons revolve?
The electrons revolve around the nucleus in circular paths.
Where is the most of the mass and all the positive charge of the atom located?
iii. Most of the mass and all the positive charge of the atom were located near the atom’s central core. He called the central core the nucleus.
Who explained the stability of the atom?
In 1913, Neil Bohr explained the stability of the atom based on the observations of his experiments. According to him:
What are the three parts of an atom?
No Comments. on 5 Chemistry Models of Atoms – Theory – Scientists – Definition. The concept of atom, especially the chemistry models of atoms, is really important in chemistry study. Atom is the tiniest of an element. Atom consists of three main parts which are proton, electron and neutron.
What are the components of an atom?
Atom Basic Concept. As mentioned previously, atom consists of three main components: proton, neutron and electron. The structure of atom consists of nucleus and electrons. Nucleus is the center of an atom where the proton and neutro n are located. Here’re brief explanations about atom components.
Why are chemical models important?
The chemical models of atoms are strongly important to understand the chemical bonding in element. Electrons play the important role in the chemical reactions. Electron transfers from one atom to another atom occur in every chemistry reactions. Chemical reactions consist of two main chemical bondings which are ionic and covalent bonding.
What is the mass of an electron?
It orbits the nucleus. Differ from proton, electron has the negative charge. The mass of electron is really small compared to proton and neutron. It has mass of approximately 9.1095 x 10 -31 kg.
Where are electrons located in an atom?
Electrons are located in the certain orbits around the atom’s nucleus. These orbitals are stable. Bohr called these parts as the stationary orbits
Which theory did Dalton use to support his theory?
He used the Antoine Lavoisier theory to support this point. The weight of atom determines the characteristic of atom. Dalton believed that all atoms in the same element must have same weights. Every single atoms in oxygen is same to another.
Which theory is most helpful to understand the chemistry study, especially the chemical models of atoms?
The nucleus finding by Rutherford is really helpful to understand the chemistry study, especially the chemical models of atom. 4. Bohr Theory. After Rutherford’s great theory, his student, Neils Bohr made the new atom model in 1913.
What are the three subatomic particles that make up an atom?
Atom is made up of 36 subatomic particles, out of which the most important are the electrons, protons, and neutrons . These three particles are called elementary fundamental particles of an atom because these are essential constituents of atoms.
Who discovered the structure of an atom?
The first evidence-based theory on the structure of the atom was given by John Dalton, known as Dalton’s Atomic theory, in the year 1808. According to this theory, the smallest and the ultimate particle of matter is known as an atom. However, towards the end of the 19 t h century and at the beginning of the 20 t h century, scientists like J.J. Thomson, Goldstein, Rutherford, Chadwick, Bohr and many more, as a result of their researchers, established that the atom is not as simple as suggested by Dalton.
What are the fundamental particles of an atom?
In this article, the structure of an atom, you have understood what an atom is, the parts of an atom, the properties of the fundamental particles of an atom, i.e., protons, electrons, and neutrons. With examples of carbon, nitrogen provided on this page, you can explain the structure of an atom. This article is also helping in recollecting the different types of atoms and their meaning.
Where are electrons located in space?
All the electrons are present in extranuclear space around the nucleus, revolve in well-defined circular paths known as orbits. The orbits are also called energy levels or energy shells, and these are designated as K, L, M, N, O, … The order of the energy of these energy shells is K < L < M < N < O, … Electrons when revolving around the nucleus do not lose or gain energy. Thus, the energy of electrons remains stationary.
What are the four forces that hold particles in an atom?
The particles are held within the atom by one of the four fundamental forces- gravity, electromagnetic force, strong force, and weak force.
Which atoms are stable?
Stable: Atoms with protons, electrons, and neutrons in balance are stable atoms.
Why do we have atoms?
Atoms are the building blocks of matter. Therefore, the existence of different kinds of matter around us is due to atoms present in them. The idea of the relativity of matter was considered run back in India around 500 B C. The Indian philosopher Maharshi Kanad postulated that if we go on the dividing matter (padarth), we get smaller and smaller particles.
What is the idea of atoms?
Though their ideas about atoms were rudimentary compared to our concepts today, they outlined the idea that everything is made of atoms, invisible and indivisible spheres of matter of infinite type and number. These scholars imagined atoms as varying in shape depending on the type of atom.
Who developed the quantum model of the atom?
At this point, many scientists were investigating and trying to develop the quantum model of the atom. Chief amongst these was Austrian physicist Erwin Schrödinger, who you’ve probably heard of before ( he’s the guy with the cat and the box ).
Why is the Bohr model incorrect?
Because the Bohr model assumes that electrons are just added as though we were just adding more and more hydrogen. This is incorrect, because things get very complicated after hydrogen, with electron orbitals, the uncertainty principle and wave functions of electrons and many other things.
What is the name of the model that explains the atom as a sphere of positive charge?
In 1904, he put forward his model of the atom based on his findings. Dubbed ‘The Plum Pudding Model’ (though not by Thomson himself), it envisaged the atom as a sphere of positive charge, with electrons dotted throughout like plums in a pudding. Scientists had started to peer into the atom’s innards, but Thomson’s model would not hang around for long – and it was one of his students that provided the evidence to consign it to history.
What is matter made of?
All matter is made up of atoms. This is something we now take as a given, and one of the things you learn right back at the beginning of high school or secondary school chemistry classes. Despite this, our ideas about what an atom is are surprisingly recent: as little as one hundred years ago, scientists were still debating what exactly an atom looked like. This graphic takes a look at the key models proposed for the atom, and how they changed over time.
What did Rutherford discover about the atomic structure?
Rutherford devised an experiment to probe atomic structure which involved firing positively charged alpha particles at a thin sheet of gold foil. The alpha particles were so small they could pass through the gold foil, and according to Thomson’s model which showed the positive charge diffused over the entire atom, the should do so with little or no deflection. By carrying out this experiment, he hoped to be able to confirm Thomson’s model, but he ended up doing exactly the opposite.
Where did the idea of atoms come from?
In fact, we have to go all the way back to Ancient Greece to find its genesis. The word ‘atom’ actually comes from Ancient Greek and roughly translates as ‘indivisible’. The Ancient Greek theory has been credited to several different scholars, but is most often attributed to Democritus (460–370 BC) and his mentor Leucippus . Though their ideas about atoms were rudimentary compared to our concepts today, they outlined the idea that everything is made of atoms, invisible and indivisible spheres of matter of infinite type and number.