
What is the most common fixative for histology?
10% neutral buffered formalin is the most common fixative for routine histology 1,2,3,11 and is suitable for immunohistochemistry (IHC) and most molecular testing if appropriately applied. What fixatives are commonly used in histology? The most commonly used fixative in histology is formaldehyde.
What is the purpose of fixation in histology?
3. The purpose of fixation is to preserve tissues permanently in as life-like a state as possible. The fixative should be 15 – 20 times more in volume then the specimen. 4.
What is the composition of fixatives?
Composition of Fixatives:-1-Formalin Solution (10%, unbuffered): Formaldehyde (37-40%) - 10 ml Distilled water - 90 ml Mix well.2-Formalin Solution (10%, buffered neutral): Formaldehyde (37-40%) - 100 ml Distilled water - 900 ml NaH2PO4 - 4.0 g Na2HPO4 (anhydrous) - 6.5 g Mix to dissolve.
What are the different types of coagulant fixatives?
Coagulant FixativesCoagulant Fixatives Alcohols (Ethanol, Methanol) Acetone Zinc Salts Mercuric Chloride Chromium Trioxide Picric acid 10. Non Coagulant FixativesNon Coagulant Fixatives Formaldehyde Gluteraldehyde Osmium Tetroxide Potassium Dichromate Acetic acid 11.
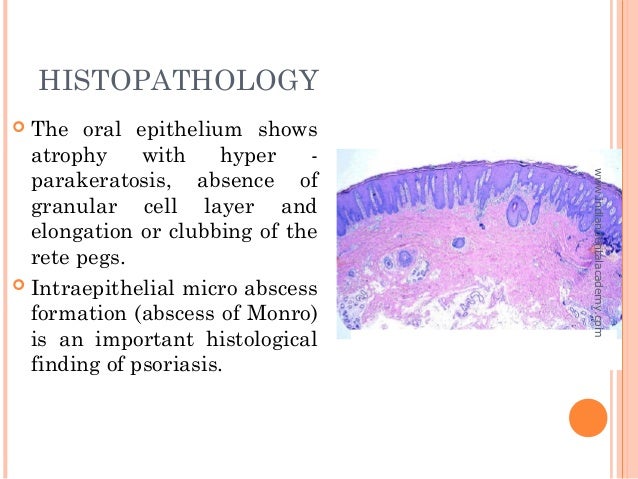
Which is the most commonly used fixative in histopathology?
Formaldehyde, by far the most popular agent used for histopathology and glutaraldehyde, widely used for ultrastructural studies requiring electron microscopy, are described here. Other reagents are discussed in Part 3. Unbuffered formalin will slowly oxidize to formic acid resulting in a fall in pH.
What are the five major fixatives?
There are five major groups of fixatives, classified according to mechanism of action:Aldehydes.Mercurials.Alcohols.Oxidizing agents.Picrates.
What is the most common fixative used?
FormalinFormalin is most commonly used fixative. It is cheap, penetrates rapidly and does not over- harden the tissues. The primary action of formalin is to form additive compounds with proteins without precipitation. Formalin brings about fixation by converting the free amine groups to methylene derivatives.
What is fixative and its types?
Fixation is considered as physiochemical process where cells or tissues are fixed chem- ically. Fixatives perform various functions such as prevention of autolysis and tissue putrefaction. Various fixative agents include formaldehyde, glutaraldehyde, osmium tetroxide, glyoxal, picric acid, and so on.
How many types of fixation are there?
three typesThere are generally three types of fixation processes depending on the sample that needs to be fixed: Heat fixation: Heat Fixation is used for the fixation of single cell organisms, most commonly bacteria and archaea.
What is Carnoy's fixative?
Carnoy's solution is a substance used as a complementary treatment after the conservative excision of odontogenic keratocyst. The application of Carnoy's solution promotes a superficial chemical necrosis and is intended to reduce recurrence rates.
Why is formalin used in histology?
The fixative 10% buffered formalin is commonly used to preserve tissues for routine histology in many labs. The formaldehyde has a greater chance for oxidation in this concentration of tissue fixative and eventually the solution will start to drop in pH, in spite of the buffer.
Why is formalin used as a fixative?
Formalin is the widely used fixative in pathology labs worldwide owing to its convenience in handling, high degree of accuracy and extreme adaptability.
What are simple fixatives?
Simple Fixatives – These fixatives are made up of simple chemical compounds and take more time for the fixation of tissues. For example, Formalin, Picric acid, Mercuric oxide, osmic acid, Osmium tetroxide etc.
Why are fixatives used?
A fixative is a stabilizing or preservative agent: Dye fixatives or mordants, are chemical substances used in processing fabrics to create circumstances in the micro-substrates causing dye molecules to adhere and remain that way.
What is ideal fixative?
An ideal fixative should: Preserve the tissue and cells as life-like as possible, without any shrinking or swelling and without distorting or dissolving cellular constituents. Prevent putrefaction by bacteria and prevent autolysis by cathepsin-containing cells.
What are cytological fixatives?
Cytological fixatives must fix and dry any smear or swab specimen quickly and reliably so that rapid staining suitable for immediate diagnosis can be achieved. The focus is on the preservation of the cytoskeleton structure and cell shapes.
What are the classification of fixatives?
Fixatives are also classified as additive and non-additive. Additive fixatives combine with molecules of the tissue so that the fixative, or some of its components, become part of the tissue, and it is present in the following steps of the histological processing.
What are simple fixatives?
Simple Fixatives – These fixatives are made up of simple chemical compounds and take more time for the fixation of tissues. For example, Formalin, Picric acid, Mercuric oxide, osmic acid, Osmium tetroxide etc.
What are the different types of fixatives used in cytology?
The most commonly used fixatives for diagnostic pathology and cytologic specimens are 10% NBF and 95% ethanol [3]. In our institution, 96% alcohol used by clinicians as a fixative for cytology specimens because of its accessibility and affordability.
What fixative means?
A fixative is a stabilizing or preservative agent: Dye fixatives or mordants, are chemical substances used in processing fabrics to create circumstances in the micro-substrates causing dye molecules to adhere and remain that way.
What is the most common fixative used in histology?
Formaldehyde (10% neutral buffered formalin) is by far the most popular fixative used in histology since it penetrates the tissue well and creates crosslinks without affecting the sample tissue’s antigenicity. While it is relatively slow to fix, it is highly recommended for immunohistochemical techniques.
What is fixation in histology?
By definition, fixation is the process of preserving biological tissues by terminating any biochemical reactions thereby preventing autolysis and putrefaction.
How does formaldehyde work?
Formaldehyde works by forming crosslinks between proteins in the sample while picric acid slowly penetrates the tissues to coagulate the proteins and form salts with basic proteins. Acetic acid counters the shrinkage caused by picric acid and coagulates nucleic acids (useful for visualizing meiotic chromosomes).
What is a good fixative for connective tissue?
Picrates satisfactorily penetrate tissue samples and are ideal fixatives for connective tissues. A good example of picrates is Bouin’s solution (a mixture composed of 40% formaldehyde, picric acid saturated aqueous solution, glacial acetic acid). Formaldehyde works by forming crosslinks between proteins in the sample while picric acid slowly penetrates the tissues to coagulate the proteins and form salts with basic proteins. Acetic acid counters the shrinkage caused by picric acid and coagulates nucleic acids (useful for visualizing meiotic chromosomes). However, it is not recommended when working with red blood cells. Bouin’s solution is explosive so take extra precaution when using it.
What is perfusion fixation?
Perfusion fixation can best be described as fixation through blood flow. The fixative is injected into the heart and spreads through the entire body.
How does fixative work?
The fixative is injected into the heart and spreads through the entire body. Since the tissue doesn’t die until it is fixed, you can get a sample with perfect morphology. Unfortunately, the subject dies during the process. Immersion fixation involves the use of fixative solutions.
What is the function of paraformaldehyde?
Paraformaldehyde (PFA) is an effective fixative that reacts with primary amines found in the protein to form crosslinks (“methylene bridges”). PFA works great in stabilizing proteins and preserving morphology, but it fixes very slowly (more than 24 hours for smaller tissues and up to a few weeks for larger tissues) and may mask antigenic sites.
What are some fixatives for histology?
Histology fixatives are not in any way restricted to these two solutions. There are many other fixatives we use every day such as methanol, acetone, glutaraldehyde, and many more. The choice depends on the starting cells or tissue and also the technique to be applied.
What is the purpose of histology fixatives?
We are all using some kind of histology fixatives in the lab, but do we actually know what it’s doing to our cells and tissues? No matter what histology fixative we use, the purpose is to immobilize antigens and retain good cellular structure to allow us to do some kind of histology analysis.
Why are antigenic sites masked?
Due to the formation of the protein cross-links during fixation, antigenic sites can be masked which can prevent recognition of the antigen by the antibody. This can be overcome by adding in an antigen retrieval step before immunohistochemistry protocols. This can be via a heat- or chemical-induced method.
Why do we fixate?
Usually we do fixation in order to do immunohistochemistry to allow us to investigate our tissue samples using antibodies. The whole process of fixation can be problematic as different epitopes require different fixation techniques and so this is yet another method that requires optimization.
What is the function of formaldehyde?
The function of formaldehyde, which cross-links proteins, is discussed above. The role of the picric acid in this solution is to slowly penetrate into the tissue and cause coagulation of proteins by forming salts with basic proteins. This can, however, cause some shrinkage of the tissue.
How long should you soak a biopsy in PFA?
Small pieces of tissue should ideally be immersed in PFA for at least 24h for a good level of preservation. Larger tissues, such as human biopsy samples, may need up to a few weeks in PFA to achieve adequate fixation.
How long can you keep Bouin's solution?
Large specimens can be kept in Bouin’s for longer, possibly up to three days, though it depends on the tissue. Due to the toxic nature of the components of Bouin’s solution, appropriate safety precautions should be taken. In particular, picric acid can be explosive and so proper disposal measures should be taken.
Why should fixatives be made up of stock solutions?
Poor quality reagents can produce poor quality fixation. Some formulated fixatives should be made up from stock solutions immediately before use because they are unstable (eg. Helly’s fluid).
When the temperature of a fixative is raised or lowered (as is sometimes recommended for particular histochemical?
When the temperature of a fixative is raised or lowered (as is sometimes recommended for particular histochemical procedures), the rate of diffusion into the specimen is affected, as is the rate of the chemical
What temperature do you fix a tissue?
For light microscopy initial fixation is usually carried out at room temperature and this may be followed by further fixation at temperatures up to 45°C during tissue processing. This is really a compromise that appears to be widely accepted to produce good quality morphological preservation. Microwave fixation may involve the use of higher temperatures, up to 65°C, but for relatively short periods. See Part 5 for further discussion.
What is the best fixative for histology?
A number of fixatives exists and the use of a particular type is dictated by the downstream analysis. For histology, the most effective and commonly used fixatives are aldehyde-based. The following fixatives are recommended for H&E staining, and most IHC markers and special stains: 1 Neutral Buffered Formalin (NBF): A 10% formaldehyde buffer solution, pH 7.0-7.4 is commonly used in most laboratories. Immediately after surgery, tissue is completely immersed in 10% NBF solution and timed. A ready-to-use solution is available from various vendors in the US. The timing of fixation determines optimal fixation as discussed further. 2 Paraformaldehyde (PFA) solution: Freshly prepared 4% PFA solution produces similar results and is cost effective. Due to its fast degradation, this solution is prepared fresh each time before use.
What is fixative in biochemistry?
Fixation is a chemical process by which biological tissue is preserved to represent the sample’s in vivo state as much as possible. In order to preserve a tissue sample in a state as near to life as possible, the postmortem processes of autolysis, which is self-degradation via proteolytic enzymes, and/or putrefaction, which is the decay of organic matter through microorganism action, must be halted. An ideal fixative should preserve the given tissue sample in a way that is representative of its in vivo situation; cellular and extracellular morphology should be preserved, and the fixative should not denature proteins that are important for histopathological analysis.
How long should fixative be exposed to a tissue sample?
A fixative should be exposed to the tissue sample for as long as is needed for the solution to completely penetrate the sample. For immersion fixation, certain factors such as density of the tissue sample, rate of penetration, and temperature must be taken into consideration. It is important to note that rate of penetration and rate of fixation are two completely different processes of a fixative, with the latter proceeding slower than the former. A general rule of thumb to apply for the rate of penetration is 1 mm/hour. A fixation time of 24 hours is recommended for NBF-treated samples.
What is the mechanism of formaldehyde?
Formaldehyde’s mechanism of fixation is through cross-linking, or creating covalent chemical bonds, between amino acid residues, mostly commonly that of amino acid lysine residues ( side chain amino groups of lysine), which result in methylene bridges. Cross-linking of formaldehyde can also occur between the aminomethylol groups and phenol, indole, and imidazole side chains. Furthermore, formaldehyde acts on a variety of amino acids, such as lysine, arginine, tyrosine, asparagine, histidine, glutamine, and serine. Cross-linking fixatives maintain internal structures of a sample and do not harm the structure of the protein significantly. The use of formaldehyde is favorable as it maintains morphology of the tissue sample and secondary and tertiary protein structure are unaffected and thus preserved. It has been proposed that formaldehyde is an effective fixative because of its fast penetration speed.
How does fixation work?
The mechanism of action of fixation is through rapidly terminating all ongoing enzymatic reactions and metabolic activities by denaturing intrinsic biomolecules. In doing this, proteolytic enzymes that would otherwise digest the tissue sample via autolysis are denatured, and autolytic processes are stopped. Fixatives also protect the sample from extrinsic damage as they are toxic to most common microorganisms (bacteria in particular) that may otherwise colonize a tissue sample. In addition, many fixatives chemically alter the treated tissue to be less palatable to opportunistic microorganisms, thereby preventing the process of putrefaction.
How long should fixative be left out of tissue?
Generally, it is not recommended to fix the tissue for more than 36 hours to avoid over-fixation. Both are problems that require their own solutions and must be avoided when fixing a tissue sample. The duration of the exposure of a sample to the fixative is thus a very important issue that must be carefully calibrated.
How long does it take to fix a tissue?
4-5 mm), and use ample amount of fixative, making sure tissue is completely immersed in the fixative. Fixation must be performed for no more than 24-36 hours depending on the size of tissue. Timing of the exposure of a sample to the fixative is important and must be calibrated.
