
Integrated Rate Laws
- First order reactions. For a first order reaction, we know that the rate of reaction is dependent on one 1st order reactant.
- 2nd order reactions. For a 2nd order reaction, we know that the rate of reaction is dependent on the square of the reactant’s concentration.
- 0th order reactions. ...
- Graphs. ...
- Example. ...
- Determine time. ...
How to determine integrated rate law?
Rate Law and Rate Constants
- Rate Constants. Therefore, the units of k (assuming that concentration is represented in mol.L -1 or M and time is represented in seconds) can be calculated via the following equation.
- Differential Rate Equations. ...
- Integrated Rate Equations. ...
- Solved Examples on the Rate Law. ...
- Recommended Videos. ...
How do you calculate rate law?
By the end of this section, you will be able to:
- Explain the form and function of a rate law
- Use rate laws to calculate reaction rates
- Use rate and concentration data to identify reaction orders and derive rate laws
How to integrate rate laws?
There are a couple of rules to writing rate expressions:
- Expressions for reactants are given a negative sign. This is because the reactant is being used up or decreasing.
- Expressions for products are positive. This is because they are increasing.
- All of the rate expressions for the various reactants and products must equal each other to be correct. ...
What is the formula for rate law?
So, based on the above formula, the ROE for Keystone Law Group is: 37% = UK£6.2m ÷ UK£17m (Based on the trailing twelve months to July 2021). The 'return' is the amount earned after tax over the last twelve months. So, this means that for every £1 of its shareholder's investments, the company generates a profit of £0.37.
What are the types of rate law?
Rate laws can be expressed either as a differential rate law, describing the change in reactant or product concentrations as a function of time, or as an integrated rate law, describing the actual concentrations of reactants or products as a function of time.
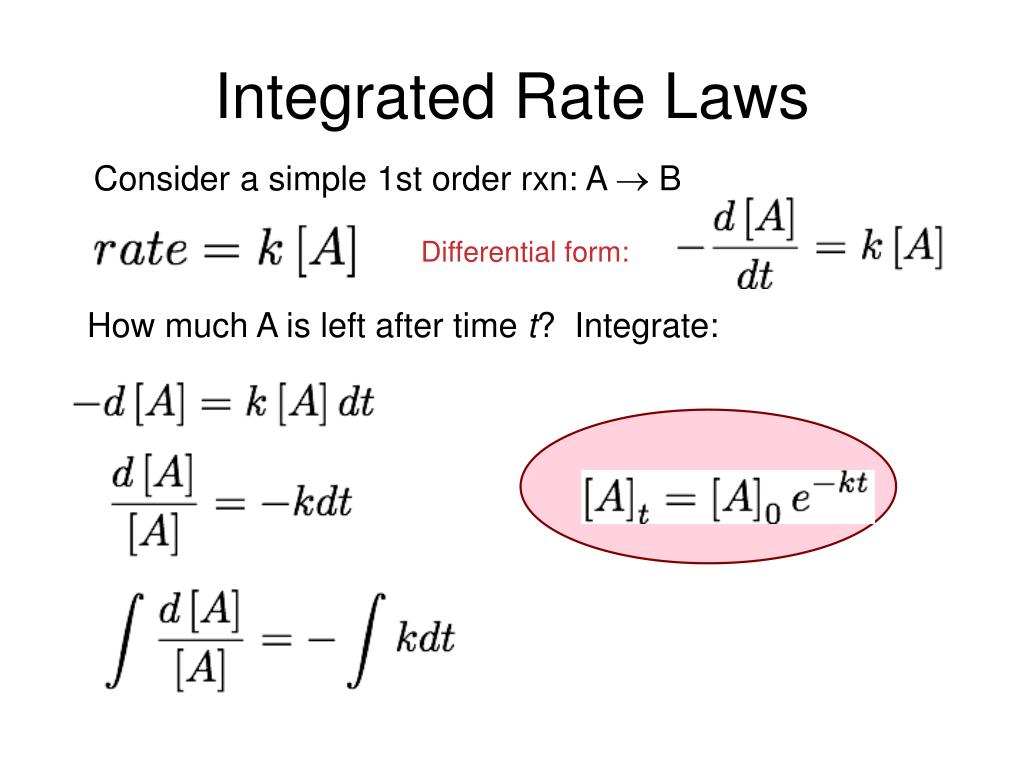
What is the first-order integrated rate law?
The integrated rate law for the first-order reaction A → products is ln[A]_t = -kt + ln[A]_0. Because this equation has the form y = mx + b, a plot of the natural log of [A] as a function of time yields a straight line.
What is integrated rate of reaction?
Hence, it is very difficult to determine the rate of reaction from the concentration-time graph. Therefore, we integrate the differential rate equation to obtain a relation between the concentration at different points and rate constant. This equation is known as integrated rate equation.
How do you derive integrated rate laws?
1:1225:59Derivations of 0th, 1st & 2nd order integrated rate law - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo if R equals K. All right so I know that the rate equals K now the rate can also equal somethingMoreSo if R equals K. All right so I know that the rate equals K now the rate can also equal something else you can also equal the negative change of my a over time certainly a is going to get used up.
What is the second-order integrated rate law?
The integrated rate law for the second-order reaction A → products is 1/[A]_t = kt + 1/[A]_0. Because this equation has the form y = mx + b, a plot of the inverse of [A] as a function of time yields a straight line. The rate constant for the reaction can be determined from the slope of the line, which is equal to k.
What is zero first and second-order reaction?
A zero-order reaction proceeds at a constant rate. A first-order reaction rate depends on the concentration of one of the reactants. A second-order reaction rate is proportional to the square of the concentration of a reactant or the product of the concentration of two reactants.
What is the purpose of integrated rate law?
The integrated rate law gives the rate of a chemical reaction as a function of the initial concentration of one or more reactants after a specific period of time.
What is the difference between rates of reaction and integrated rate laws?
Differential Rate Law can say how long it's going to take for the whole reaction to occur, but the Integrated Rate Law talks about the reaction at a specific moment in time.
How do you write a rate law?
A rate law relates the concentration of the reactants to the reaction rate in a mathematical expression. It is written in the form rate = k[reactant1][reactant2], where k is a rate constant specific to the reaction.
What is the use of integrated rate equation?
We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate the time required for a reaction to proceed to a certain extent.
What is initial rate of reaction?
The initial rate of a reaction is the instantaneous rate at the start of the reaction (i.e., when t = 0). The initial rate is equal to the negative of the slope of the curve of reactant concentration versus time at t = 0. Top.
What is the integrated rate equation for zero-order reaction?
The integrated rate law for the zero-order reaction A → products is [A]_t = -kt + [A]_0. Because this equation has the form y = mx + b, a plot of the concentration of A as a function of time yields a straight line. The rate constant for the reaction can be determined from the slope of the line, which is equal to -k.
What is the rate of reaction formula?
The rate of a chemical reaction can also be measured in mol/s. For example, if two moles of a product were made during ten seconds, the average rate of reaction would be 2 ÷ 10 = 0.2 mol/s.
First Order Reactions
2nd Order Reactions
- For a 2nd order reaction, we know that the rate of reaction is dependent on the square of the reactant’s concentration.
0th Order Reactions
- A 0th order reaction rate is defined by the decrease in concentration of reactants over time. A zeroth order reaction rate does not depend on the concentration of the reactant, it means that the rate of reaction is constant over time even though the reactants are decreasing as the reaction progresses. The differential rate law:
Graphs
- The most useful aspect of the integration is to arrive at an equation y = mx + c as this can be easily graphed. And by distinguishing the patterns of the graphs, we can quickly determine if the reaction is 0th, 1st, or 2nd order.
Example
- Given the experimental data (see table below), determine the rate law for the reaction with respect to reactant A. You can determine the order from integrated rate law. Calculate the values of ln[A] and 1/[A] . Plot the graphs. Since graph ln[A] vs timegives a straight line and the others do not, we know that the reaction is a first order reaction with respect to A.
Determine Time
- How long will it take for the reaction to reach 0.0200 M which has a rate constant of k = 3.5 x 10⁻³ s⁻¹. The initial concentration is 0.100 M. To determine t, we need to know: [A]₀ – the initial concentration [A]t – the final concentration The order of the reacton The rate constant k is given 3.5 x 10⁻³ s⁻¹