
What is a Lead Acid Battery?
- Electrolyte. The electrolyte - which is a mixture of water and sulfuric acid - is a critical part of any lead acid battery.
- Plates. As mentioned above, lead plates are a key component to the construction the lead acid battery and have been since they were first invented.
- Tubular. ...
- Separator s. ...
How do you repair a lead acid battery?
Identify the bad cells :-
- Charge your batteries beforehand for at least 12 hours. After that, disconnect batteries from chargers and allow them to rest for 10 minutes.
- Open up all battery caps. ...
- Fill up each compartment to within optimum water levels. ...
- Measure the terminal voltage of battery (12v). ...
- Measure the voltage of each cell and identify the cells with lower voltages. ...
Which acid is used in lead acid battery?
The first type of rechargeable battery was, in fact, a lead-acid one. It consists of a negative electrode made of porous lead in a plate dipped in dilute sulfuric acid, where the reactions take place for the charge and discharge to occur.
What is inside an AAA battery?
With the coupon being offered right now, you can get 28 Duracell AA batteries for just $9.79 ($10.33 after-tax ... you can now have Amazon drivers leave your package inside your garage by signing up for Amazon Key. Consumer Reports has recommended ...
Can You rejuvenate a lead acid battery?
You can de-sulfate your lead-acid battery and rejuvenate it fairly easily. Since all lead-acid batteries essentially use the same principles, the only real difference between maintenance-free and sealed lead acid batteries compared to more basic filler cap models is that they have hidden caps that will need to be removed before you can revive them.
See more
What are the components of battery acid?
What Is The Formula For Battery Acid? Battery acid doesn't have a specific formula, but it's usually just composed of sulphuric acid (H2SO4) and water (H2O), with an approximate pH level of 0.8 at a 4-5 mol/L concentration.
What are the 2 main parts of a battery?
Anode - negative side of the battery. Cathode - positive side of the battery. Electrolyte - a chemical paste that separates the anode and cathode and transforms chemical energy into electrical energy.
What are the three components used to generate electricity in a lead acid battery?
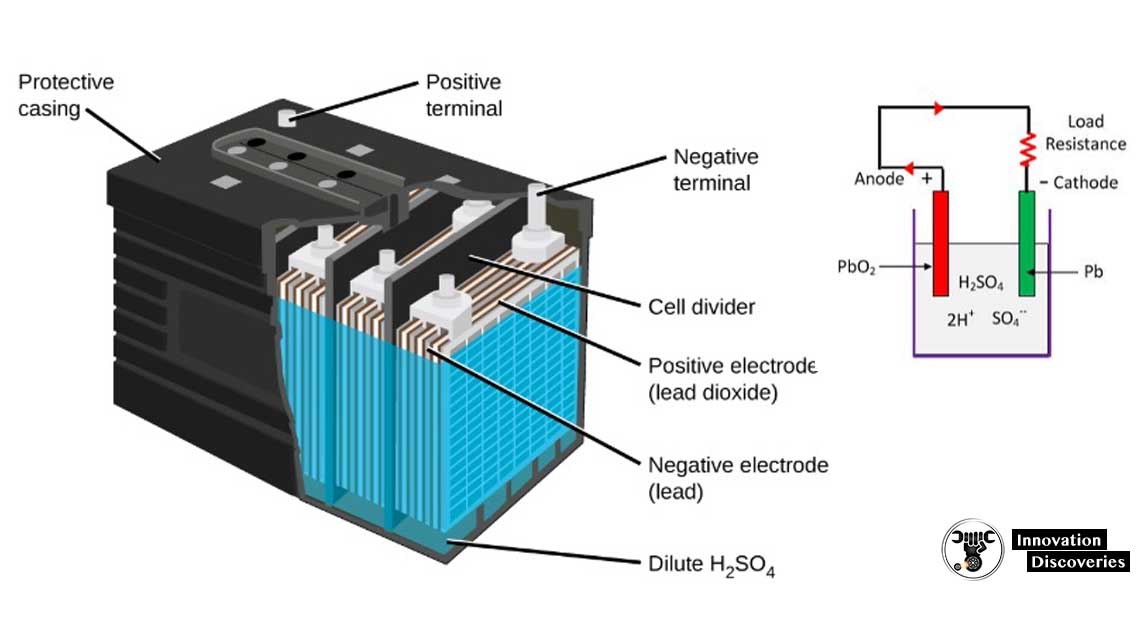
5.2. In a lead-acid battery, the cathode is made of lead-dioxide, and the anode is made of metallic lead. The two electrodes are separated by an electrolyte of sulfuric acid. As the battery charges, the sulfuric acid reacts with the lead in the anode and cathode to produce lead sulfate.
What is in between the plates of a lead acid battery?
Lead acid batteries use positive and negative plates, submerged in electrolyte. The reaction between the lead plates and the electrolyte generates the power.
What are the 5 parts of a battery?
What are the parts of a battery? Seven different components make up a typical household battery: container, cathode, separator, anode, electrodes, electrolyte, and collector.
What are the four components of a battery?
Li-ion batteries consist of largely four main components: cathode, anode, electrolyte, and separator. Every single component of a Li-ion battery is essential as it cannot function when one of the components is missing.
What are plates in batteries?
Battery Plates: The element consists of stacked alternating positive and negative plates. The plates are connected at the top by a cast-on strap that is welded to the plates. The elements fit into the individual cells of each battery.
What are the plates in a battery made of?
lead platesA battery cell consists of two lead plates a positive plate covered with a paste of lead dioxide and a negative made of sponge lead, with an insulating material (separator) in between.
How many plates are there in lead acid battery?
two flatAll lead-acid batteries consist of two flat plates—a positive plate covered with lead dioxide and a negative made of sponge lead—that are immersed in a pool of electrolyte (a combination of sulfuric acid (35%) and water solution (65%). Electrons are produced from the chemical reaction producing voltage.
What is a battery and explain the main parts of battery?
In chemistry, batteries are made up of electrical cells, that convert chemical energy to electrical energy. The three main parts of batteries are anode, cathode and electrolyte. The two terminals, Anode and cathode, are separated by an electrolyte.
What is cathode and anode?
The Anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. The Cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction.
What are the ends of a battery called?
The cathode and anode (the positive and negative sides at either end of a traditional battery) are hooked up to an electrical circuit. The chemical reactions in the battery causes a build up of electrons at the anode. This results in an electrical difference between the anode and the cathode.
What is the container of a lead acid battery?
1. Container – The container of the lead acid battery is made of glass, lead lined wood, ebonite, the hard rubber of bituminous compound, ceramic materials or moulded plastics and are seated at the top to avoid the discharge of electrolyte. At the bottom of the container, there are four ribs, on two of them rest the positive plate and ...
What is the plate of a lead acid cell?
2. Plate – The plate of the lead-acid cell is of diverse design and they all consist some form of a grid which is made up of lead and the active material. The grid is essential for conducting the electric current and for distributing the current equally on the active material. If the current is not uniformly distributed, then the active material will loosen and fall out.
What is the active material of lead peroxide?
Lead peroxide (PbO2) – It forms the positive active material. The PbO 2 are dark chocolate broom in colour.
What is the active material of a cell?
Active Material – The material in a cell which takes active participation in a chemical reaction (absorption or evolution of electrical energy) during charging or discharging is called the active material of the cell. The active elements of the lead acid are. Lead peroxide (PbO2) – It forms the positive active material.
Which ions are positively charged?
The hydrogen ions being positively charged moved towards the cathodes and receive two electrons from there and form a hydrogen atom. The hydrogen atom reacts with lead sulphate cathode forming lead and sulfuric acid according to the chemical equation.
How many ribs are in a battery container?
At the bottom of the container, there are four ribs, on two of them rest the positive plate and the others support the negative plates. The prism serves as the support for the plates and at the same time protect them from a short-circuit.The material of which the battery containers are made should be resistant to sulfuric acid, ...
What are the two types of plates used in batteries?
The plates of the battery are of two types. They are the formed plates or plante plates and pasted or faure plates. Plante’s plates are used largely for stationary batteries as these are heavier in weight and more costly than the pasted plates.
What are the different types of lead acid batteries?
There are three common types of lead acid battery: Flooded. Gel. Absorbent Glass Mat (AGM) Note that both Gel and AGM are often simply referred to as Sealed Lead Acid batteries. The Gel and AGM batteries are a variation on the flooded type so we’ll start there.
Why are lead acid batteries better than wet cells?
The way electrolyte is stored in a sealed lead acid battery means that they have a number of advantages over the older wet cell/flooded design: There is no liquid to spill or leak so the batteries are easier to ship and can be mounted at angles. They are better at delivering power.
How long do SLA batteries last?
causing burn out in alternators designed to charge flooded cell batteries. shorter lives – The standard lifespan for SLA batteries is three to five years; for wet-cell batteries it’s up to 20 years. There is also a small difference between AGM and Gel during their lives.
How much energy does a flood battery use?
Flooded batteries convert 15-20% of electrical energy from a charger into heat instead of stored power, Gel cells convert 10-16% while the best AGMs lose just 4%. The design of the Absorbent Glass matt (AGM) in the the sealed lead acid battery allows for faster charge times. Because the glass matt absorbs and immobilises ...
What is a cell connector?
Cell connectors which join the positive strap of one cell to the negative strap of the next cell. Electrolyte – either as a solution of water and sulfuric acid or a gel. A case and lid – normally made from a polypropylene plastic. Terminal posts (usually lead) to connect the battery to an appliance.
Why is the battery case deeper than the lead plate?
This is because as the battery is used bits of the active material (paste) fall off the plates and build up at the bottom of the battery. If there was no space for them to do so they would cause a connection between the positive and negative plates, the cell would short out and the battery would die.
How much does a flood battery discharge?
They discharge at a lower rate when not in use. Flooded batteries discharge at a rate of about 1% per day compared to 1-3% per month from sealed lead acid units.
Where Does a Lead-acid Battery use in?
It is mostly used in vehicles such as automobiles, forklifts, dump trucks, golf cars, ambulances, and even uninterruptible power supplies used in computers.
When was the first battery invented?
A lead-acid battery was invented by the French physician Gaston Planté in 1859. This might be an old invention but it still widely-used until now. It is also the first rechargeable battery among all of the batteries. A few of the reasons why it is still in use today because it is inexpensive, reliable, tolerant to overcharging, can be recycled and has many sizes and capacities available worldwide.
What is sulfuric acid?
Sulfuric Acid – a strong acid made by oxidizing solutions of sulfur dioxide. It is used as an electrolyte.
What is lead dioxide used for?
Lead Dioxide (PbO2) – is an oxidizing agent used in the manufacture of dyes, matches, and rubber substitutes. It forms the positive active material.
Is lead sulfate soluble in alcohol?
It is synthesized by adding Sulfuric acid to a lead salt solution. It is slightly soluble in hot water but insoluble in alcohol.
How many plates are in a battery?
The batteries are categorised according to the number of plates i.e. 15 plates, 17 plates and 19 plates, etc.
How to store a battery?
For storage, charge the battery, remove the electrolyte, dry the battery and keep it in cool dry place after reassembling.
What is the color of the positive plate in H2S04?
Chemical action. While the cell is fully charged the positive plate is of dark chocolate brown and negative of grey colour. In Case Of discharging the H2S04 decomposes into its ions the hydrogen and sulphate.
What are the two types of plates?
Plates. There are two types of the plates the positive plate and negative plate. The active material of the positive plate is Pb02 (lead peroxide) and spongy lead for negative plate. According to the construction the plates are divided into the followings:
Can you bring a flame near a battery?
Never bring na ked flame near the battery while charging the battery and room should be well ventilated.
