
What the primary physician should know about tuberculosis?
- The TB skin test is performed by injecting a small amount of fluid (called tuberculin) into the skin on the lower part of the arm.
- A person given the tuberculin skin test must return within 48 to 72 hours to have a trained health care worker look for a reaction on the arm.
- The result depends on the size of the raised, hard area or swelling.
How to tell if you have tuberculosis?
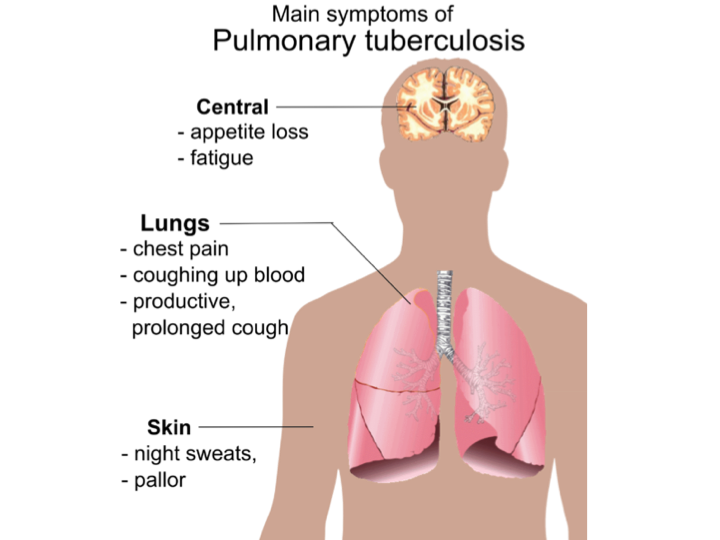
The most common symptoms of TB are:
- a cough for three weeks or longer
- weight loss
- loss of appetite
- high temperature or fever
- night sweats
- extreme tiredness or lack of energy.
What are some interesting facts about tuberculosis?
This includes:
- individuals who work in institutions or facilities which house people who are at high risk for this condition, like – homeless shelters, hospitals, nursing homes, correctional facilities, and residential homes ...
- seniors;
- children have a higher risk of developing the most severe forms of TB;
- people living with HIV infection;
What are facts about tuberculosis?
Tuberculosis complications include:
- Spinal pain. Back pain and stiffness are common complications of tuberculosis.
- Joint damage. Arthritis that results from tuberculosis (tuberculous arthritis) usually affects the hips and knees.
- Swelling of the membranes that cover your brain (meningitis). ...
- Liver or kidney problems. ...
- Heart disorders. ...
See more
What is tuberculosis write its symptoms and pathophysiology?
Tuberculosis (TB) is caused by a type of bacterium called Mycobacterium tuberculosis. It's spread when a person with active TB disease in their lungs coughs or sneezes and someone else inhales the expelled droplets, which contain TB bacteria.
What are the pathologic changes of tuberculosis?
Arthur Dannenberg described the pathology of tuberculosis in detail [2, 3]. There are five stages: onset, symbiosis, early stages of caseous necrosis, interplay of cell-mediated immunity and tissue damaging delayed-type hypersensitivity, and liquefaction and cavity formation.
What pathophysiology means?
Definition of pathophysiology : the physiology of abnormal states specifically : the functional changes that accompany a particular syndrome or disease.
What are the 3 stages of tuberculosis?
There are 3 stages of TB—exposure, latent, and active disease. A TB skin test or a TB blood test can diagnose the disease. Treatment exactly as recommended is necessary to cure the disease and prevent its spread to other people.
What are the 4 stages of TB?
TB infection happens in 4 stages: the initial macrophage response, the growth stage, the immune control stage, and the lung cavitation stage. These four stages happen over roughly one month.
What are the complications of tuberculosis?
ComplicationsSpinal pain. Back pain and stiffness are common complications of tuberculosis.Joint damage. Arthritis that results from tuberculosis (tuberculous arthritis) usually affects the hips and knees.Swelling of the membranes that cover your brain (meningitis). ... Liver or kidney problems. ... Heart disorders.
How is tuberculosis diagnosed in the microbiology lab?
The Mantoux tuberculin skin test (TST) or the TB blood test can be used to test for M. tuberculosis infection. Additional tests are required to confirm TB disease. The Mantoux tuberculin skin test is performed by injecting a small amount of fluid called tuberculin into the skin in the lower part of the arm.
What is the pathology of pneumonia?
Pneumonia is an infection that inflames the air sacs in one or both lungs. The air sacs may fill with fluid or pus (purulent material), causing cough with phlegm or pus, fever, chills, and difficulty breathing. A variety of organisms, including bacteria, viruses and fungi, can cause pneumonia.
How do neutrophils help tuberculosis?
LPS (50μg/ml) was administered intratracheally to male Fischer rats, which were then infected with M. tuberculosis via an airborne route. Intratracheal injection of LPS significantly blocked the development of pulmonary granulomas and significantly reduced the number of pulmonary colony-forming units (CFU). Treatment with amphotericin B (an LPS inhibitor) or neutralizing anti-rat neutrophil antibody reversed the development of pulmonary lesions. LPS-induced transient neutrophilia prevented early mycobacterial infection. The timing of LPS administration was important. When given intratracheally at least 10 days after aerial infection, LPS did not prevent the development of tuberculosis. Neutrophils obtained by bronchoalveolar lavage killed M. tuberculosis bacilli. These results indicate clearly that neutrophils participate actively in defense against early-phase tuberculosis.
Where are TB droplets deposited?
Infectious droplet nuclei are deposited in the alveolar spaces of the contact person where Mycobacterium tuberculosis (M. tb) can be phagocytosed by alveolar macrophages, epithelial cells, dendritic cells (DC) and neutrophils [ 8, 9 ]. Alveolar macrophages and DC are then believed to transport M. tb to local lymph nodes where T cell activation occurs and expand. Activation of the phagocytic host cell is much required to limit growth of M. tb; as in the absence of activation, disease outcome is extremely poor. Effective phagocyte activation requires a specific cellular response, as infected hosts lacking specific components of the acquired response have a poor outcome [ 10 ]. While acquired cellular protection is expressed rapidly following systemic challenge with M. tb, it is less rapid in the lung. Slow expression of protection in the lung allows mycobacteria to grow and modulate the infection site. Until recently it has not been clear whether the slow response to aerosol delivery of bacteria resulted from limited availability of antigen or inhibition of antigen-presentation by M. tb. Several studies show that the first T cell activation occurs in the draining lymph node (DLN) of the lung 8–10 days following initial challenge. The activation of T cells correlated temporally with the arrival of bacteria and availability of antigen in the DLN, however conditions for T cell activation were unique to the draining lymph nodes as the presence of antigen-producing bacteria in the lung and spleen did not result in initial activation of T cells [ 11, 12 ]. While delivery of lipopolysaccharide (LPS) to the MTB-infected lung failed to accelerate T cell priming [ 11 ], increasing the bacterial dose did accelerate the response modestly suggesting that both antigen burden and refractory cells serve to slow the response. So, protective memory cells will not become activated until they see antigen, i.e. more than 8 days post infection. Once T cells become activated they differentiate into effector T cells that migrate to the lung. By day 14 of infection, when activated T cells first arrive in the lung, bacteria are within alveolar macrophages, myeloid DC and neutrophils [ 11 ]. T cells can recognize antigen within the mycobacterially-infected lung but the antigen presentation is not optimal. It takes time for the protective T cells to reach sufficient numbers to stop bacterial growth. T cells can be divided into two subsets, Th1 and Th2, on the basis of the cytokines they produce. In tuberculosis, Th1 plays a major role in defense against tuberculosis. Th1 cells suppress Th2 cells. CD4 + T cells have unambiguously been identified as the most important lymphocyte subset for mediating protection.CD4 T lymphocytes differentiate in the peripheral tissues to adopt a variety of fates such as the Th-1 cells, which produce interferon (IFN)- γ to down-regulate Th2 responses and Th-2 cells, which produce interleukin (IL)-4. CD8 T lymphocytes produce predominantly IFN- γ. Though CD4 response is greater than the CD8 response, the latter can provide protection in the absence of CD4 help [ 13 ]. During active TB there is a local pulmonary immune response characterized by α/β T cells and strongly enhanced M. tuberculosis antigen-specific Th1 responses, with large amounts of locally secreted IFN- γ [ 14 ].
What stage of necrosis is the number of viable bacilli stationary?
In stage 3 , the stage at which caseous necrosis first occurs, the number of viable bacilli becomes stationary because their growth is inhibited by the immune response to tuberculin-like antigens released from bacilli. Stage 4 is the stage that usually determines whether the disease becomes clinically apparent.
What is the stage of symbiosis?
In stage 2 , bacilli grow logarithmically within the immature nonactivated macrophages. These macrophages enter a tubercle from the bloodstream. This stage is termed symbiosis because bacilli multiply locally without apparent damage to the host, and macrophages accumulate and divide.
Why does paratracheal lymphadenopathy occur?
Associated paratracheal lymphadenopathy may occur because the bacilli spread from the lungs through the lymphatic system. Active tuberculosis develops in only 5% to 10% of persons exposed to M. tb. Fig. 2 shows typical chest X-ray before (A) and after (B) chemotherapy.
What is stage 4 of cytotoxic disease?
Stage 4 is the stage that usually determines whether the disease becomes clinically apparent. Cell-mediated immunity plays a major role in this situation. The cytotoxic delayed- type hypersensitivity immune response kills these macrophages, causing enlargement of the caseous center and progression of the disease.
What are the two subsets of T cells?
It takes time for the protective T cells to reach sufficient numbers to stop bacterial growth. T cells can be divided into two subsets, Th1 and Th2, on the basis of the cytokines they produce. In tuberculosis, Th1 plays a major role in defense against tuberculosis. Th1 cells suppress Th2 cells.
What is TB in humans?
Tuberculosis (TB) is a chronic inflammatory disease caused by the pathogenic bacterium Mycobacterium tuberculosis. A wide variety of host- and pathogen-associated variables influence the clinical manifestation of TB in different individuals within the human population. As a consequence, the characteristic granulomatous lesions ...
Can TB be reproduced in animals?
In particular, post-primary TB, which accounts for the majority of cases of active TB and is responsible for transmission between individuals via aerosol exposers, cannot be reproduced in animals and therefore cannot be adequately modeled experimentally.
What is the role of immunopathogenesis in TB?
of the immunopathogenesis of TB can facilitate the design of effective vaccines, new drug candidates and evaluation of their efficacy [53]. Understanding latent tuberculosis can also be the key to improve diagnostic and novel treatment strategies [54].
What is the effect of NK cells on M. tuberculosis?
tuberculosis. Rohan Dhiman et al. also found that IL-22 can restrict growth of M. tuberculosis in macrophages by enhancing phagolysosomal fusion [39]. Nonetheless to fully understand the importance of NK cells in M. tb infection it may be necessary to differentiate their contributions at different stages of disease. Certain T subsets, such as NKT cells and γδ T cells, have features of innate immune cells including a partially activated phenotype, a rapid response following detection of infected cells, and the modulation of other cell types. Together with NK cells, these cell subsets are functionally defined as innate lymphocytes.CD1d-restricted invariant NKT (iNKT) cells are a conserved subset of T cells that express an invariant T cell receptor (TCR) α chain (Vα24-Jα18 in humans, and Vα14-Jα18 in mice) paired with TCR β chains encoded by one or a few Vβ gene segments (Vβ11 in humans, and predominantly Vβ2, 7 and 8 in mice). These cells show different phenotypes and functions [40].Many iNKT cells are CD4+, and they have been mainly associated with the induction of Th2 cytokines such as IL-4, IL-5, IL-13. This subset is believed to play a prominent role in suppression of autoimmune or chronic inflammatory diseases, and in promoting allergic conditions such as asthma. Few iNKT cells are CD8+, and most of those express only the CD8α subunit, which means that they likely express only CD8αα homodim‐ ers. An additional fraction of iNKT cells are negative for both CD4 and CD8 (DN T cells). They have been found to produce predominantly IFN-γ and other Th1-associated cytokines. Studies of human iNKT cells have shown that they have the ability to kill M. tuberculosis organisms within infected macrophages, possibly through their production of the peptide granulysin [41]. Jin S. Im et al. [42] found that the percentages of iNKT cells among total circulating T cells in TB patients were not significantly different compared to those in healthy controls. However, TB patients showed a selective reduction of the proinflammatory CD4−CD8− (DN) iNKT cells with a proportionate increase in the CD4+ iNKT cells. The mouse model of tuberculosis has been used by Sada-Ovalle et al to find that iNKT cells have a direct bactericidal effect on M. tuberculosis, and protect mice against aerosol M.TB infection [43]. Their activation requires CD1d expression by infected macrophages as well as IL-12 and IL-18. In addition, pharmaco‐ logical activation of iNKT cells with the synthetic ligand aGalCer often enhances host resist‐ ance to infection. iNKT cell use several mechanisms to modify host immunity. These include induction of DC maturation, secondary activation of effector cells (NK cells) or recruitment of inflammatory cells to the site of infection [44, 45]. Thus, by being an early producer of IFN-γ and suppressing intracellular bacterial growth, iNKT cells function as an important part of the early immune response against M. tb that affect both the innate and the adaptive arms of the immune response. Antigen-specific γ/δ T cells represent an early innate defense that may play a role in antimy‐ cobacterial immunity. Studies done in humans and animal models have demonstrated complex patterns of γ/δ T cell immune responses during early mycobacterial infections and chronic TB. Like α/β T lymphocytes, γ/δ T cells carry antigen TCR that vary in the physical properties of their ligand-binding sites. γ/δ T cells are frequently activated by a variety of pathogens including M. tb [46]. Mice lacking γ/δ T cells succumb more rapidly than control
What happens to the bacilli in stage 5?
If good cell-mediated immunity develops, a mantle of highly activated macrophages surrounds the caseous necrosis. In stage 5, bacilli evade host defenses. When liquefaction of the caseous center occurs, the bacilli multiply extracellularly, frequently attaining very large numbers. The high local concentration of tuberculin-like products derived from these bacilli causes a tissue-damaging delayed-type hypersensitivity response that erodes the bronchial wall, forming a cavity.
Where are TB droplets deposited?
Infec‐ tious droplet nuclei are deposited in the alveolar spaces of the contact person where Myco‐ bacterium tuberculosis (M. tb) can be phagocytosed by alveolar macrophages, epithelial cells, dendritic cells (DC) and neutrophils [8, 9]. Alveolar macrophages and DC are then believed to transport M. tb to local lymph nodes where T cell activation occurs and expand. Activa‐ tion of the phagocytic host cell is much required to limit growth of M. tb; as in the absence of activation, disease outcome is extremely poor. Effective phagocyte activation requires a specific cellular response, as infected hosts lacking specific components of the acquired re‐ sponse have a poor outcome [10]. While acquired cellular protection is expressed rapidly
Is TNF a regulator of T cells?
IFN-γ and TNF have long been implicated as regulators of T cell responses in mycobacterial disease [29]. The technique of gene targeting (knockout) has swept through biomedical research. IFN-γ, TNF-α, IRF-1, NF-IL6, NF-κB p50, STAT 1 and STAT 4 knockout mice succumbed to M. tuberculosis infection over time. There appears to be a cytokine and tran‐ scription factor hierarchy in experimental tuberculosis. The results indicate that these mole‐ cules play major roles in defense against the disease, IFN-γ and TNF-α being the leading players in this respect [30]. The role of neutrophils in the development of tuberculosis remained unknown for a long time. We utilized LPS-induced transient neutrophilia in the lungs [31]. LPS (50μg/ml) was admin‐ istered intratracheally to male Fischer rats, which were then infected with M. tuberculosis via an airborne route. Intratracheal injection of LPS significantly blocked the development of pulmonary granulomas and significantly reduced the number of pulmonary colony-forming units (CFU). Treatment with amphotericin B (an LPS inhibitor) or neutralizing anti-rat neutrophil antibody reversed the development of pulmonary lesions. LPS-induced transient neutrophilia prevented early mycobacterial infection. The timing of LPS administration was important. When given intratracheally at least 10 days after aerial infection, LPS did not prevent the development of tuberculosis. Neutrophils obtained by bronchoalveolar lavage killed M. tuberculosis bacilli. These results indicate clearly that neutrophils participate actively in defense against early-phase tuberculosis. Natural killer (NK) cells are innate lymphocytes which are a first line of defense against infection. NK cells can kill autologous infected cells without prior sensitization, and are believed to play a pivotal role in innate immunity to microbial pathogens. In mouse model, NK cells are activated and produce IFN-γ during the early response to pulmonary tuberculosis [31] and NK cell-produced IFN-γ regulates the anti-mycobacterial resistance mediated by neutrophils [32]. However animal models do not give a clear answer to whether NK cells is important in M. tb infection in vivo. Depletion of NK cells had no effect on bacterial replication in the lung of immunocompetent mice [33], suggesting that NK cells may be redundant in the presence of intact adaptive immunity. Surprisingly, IFN-γ knockout mice, which are impaired in their ability to clear mycobacteria, cleared them as effectively as wild-type mice when NK cells were depleted, suggesting that NK cells can inhibit protective immunity [34]. Human NK cells use the NKp46, the natural cytotoxicity receptors (NCRs) and NKG2D receptors to lyse M. tuberculosis-infected monocytes and alveolar macrophages [35], through damage of infected cells and secretion of cytokines, such as IFN-γ [36]. Inhibitory receptors of NK cells include killer immunoglobulin-like receptors (KIRs) and the NKG2A:CD94 dimer and NK cell activation can also be triggered by loss of inhibitory ligands from the cell surface. In addition, NK cells can also be activated by cytokines, including type I interferons, IL-12 and IL-18. NK cells are a potent and early source of cytokines, particularly IFN- γ, but they can also produce Th2-associated cytokines, such as IL-5 and IL-13, and the regulatory cytokine IL-10 [37]. NK cell NKp46 expression and cytotoxicity are reduced in freshly isolated peripheral blood mononuclear cells (PBMCs) from tuberculosis patients, which may be attributable to
Can mice carry TB?
A wide variety of animal models have been used to test new vaccines and drugs [15]. Mice can harbor high numbers of M. tb within lung tissue without showing clinical signs [16]. Mice do not cough nor form cavitary lesions, making them a poor model for transmission studies [17]. Fibrous capsules are not observed histologically, which can affect the validity of antibiotic studies, as M. tb would be more easily accessed by drugs in the mouse lung. In addition, because of their short life span, mice are poor models for the study of latent infection. Rat TB also showed similar pathophysiology to murine TB [31]. Guinea pigs develop robust DTH response to mycobacterial antigens and, after infection with M. tb, reproduce many of the aspects of human infection, such as caseous and mineralized granulomas, primary and hemato-genous pulmonary lesions, fibrous capsule formation, and dissemination [19],
Is tuberculosis a public health problem?
Tuberculosis is an international public health problem. It is becoming evident that M. tbinfection is a dynamic state with a wide spectrum of pathology. An improved understandingof the immunopathogenesis of TB can facilitate the design of effective vaccines, new drugcandidates and evaluation of their efficacy [53]. Understanding latent tuberculosis can also bethe key to improve diagnostic and novel treatment strategies [54].
What are the stages of tuberculosis?
According to a study conducted by Knechel, the progression of tuberculosis has several stages. 1. Latent Tuberculosis – It is the stage of infection when the person who had been exposed to the M. tuberculosis nuclei does not manifest signs and symptoms of the disease and do not have the capacity to infect other people.
What is the most fatal location of tuberculosis?
The most fatal location is the central nervous system and its infection to the bloodstream.
Is primary pulmonary tuberculosis asymptomatic?
Primary Pulmonary Tuberculosis – Since the most immediate location of pathogenesis of the organism is in the lungs, primary activation of disease in the pulmonary cavity is considered. It is usually asymptomatic and only identified through significant diagnostic examinations.
Is tuberculosis a secondary infection?
It can be considered as primary or secondary infection depending on recovery of the client from the communicable infection. It is a reportable communicable disease and a repeated exposure to it causes a person to acquire it. According to a study conducted by Knechel, the progression of tuberculosis has several stages. 1.
Where is primary tuberculosis located?
This primary TB is usually localized to the middle portion of the lungs, and this is known as the Ghon focus of primary TB. In most infected individuals, the Ghon focus enters a state of latency. This state is known as latent tuberculosis.
What is secondary tuberculosis?
Most people who develop tuberculosis, do so after a long period of latency (usually several years after initial primary infection). This is known as secondary tuberculosis. Secondary tuberculosis usually occurs because of reactivation of latent tuberculosis infection. The lesions of secondary tuberculosis are in the lung apices. A smaller proportion of people who develop secondary tuberculosis does so after getting infected a second time (re-infection).
What is the appearance of granuloma in tuberculosis?
The appearance of the granuloma in tuberculosis has been described as caseous or cheese-like on gross examination. This is principally explained by the rich mycolic acid content of the mycobacterium cell well. Because of this unique quality, the term caseous or caseating necrosis has been used to described granulomatous necrosis caused by mycobacteria tuberculosis.
What is the most common cause of TB?
Tuberculosis (TB) is a human disease caused by Mycobacterium tuberculosis. It mainly affects the lungs, making pulmonary disease the most common presentation. Other commonly affected organ systems include the respiratory system, the gastrointestinal (GI) system, the lymphoreticular system, the skin, the central nervous system, the musculoskeletal system, the reproductive system, and the liver. In the past few decades, there has been a concerted global effort to eradicate tuberculosis. Despite the gains in tuberculosis control and the decline in both new cases and mortality, it still accounts for a huge burden of morbidity and mortality worldwide. This activity reviews the evaluation and management of tuberculosis and highlights the role of interprofessional team members in collaborating to provide well-coordinated care and enhance outcomes for affected patients.
What percentage of MDR-TB is acquired?
Seventy-five percent of MDR-TB is considered primary MDR-TB, caused by infection with MDR-TB pathogens. The remaining 25% are acquired and occur when a patient develops resistance to treatment for tuberculosis. Inappropriate treatment for tuberculosis because of several factors such as antibiotic abuse; inadequate dosage; incomplete treatment is the number one cause of acquired MDR-TB.
How does the body determine the ability to eliminate an inoculum?
The body's ability to effectively limit or eliminate the infective inoculum is determined by the immune status of the individual, genetic factors and whether it is a primary or secondary exposure to the organism. Additionally, M. tuberculosispossesses several virulence factors that make it difficult for alveolar macrophages to eliminate the organism from an infected individual. The virulence factors include the high mycolic acid content of the bacteria outer capsule, which makes phagocytosis to be more difficult for alveolar macrophages. Furthermore, some of the other constituents of the cell wall such the cord factor may directly damage alveolar macrophages. Several studies have shown that mycobacteria tuberculosis prevents the formation of an effective phagolysosome, hence, preventing or limiting the elimination of the organisms.
Which countries have the most tuberculosis deaths?
The bulk of the global burden of new infection and tuberculosis death is borne by developing countries with 6 countries, India, Indonesia, China, Nigeria, Pakistan, and South Africa, accounting for 60% of TB death in 2015, (WHO, 2017) [4].
What is TB in the brain?
When TB occurs in the tissue surrounding the brain or spinal cord, it is called tuberculous meningitis. Tuberculous meningitis is often seen at the base of the brain on imaging studies. Symptoms include headache, decreased level of consciousness, and neck stiffness. The duration of illness before diagnosis is variable and relates in part to the presence or absence of other sites of involvement. In many cases, patients with meningitis have abnormalities on a chest radiograph consistent with old or current TB, and often have miliary TB.
Where does TB occur?
Extrapulmonary TB disease occurs in places other than the lungs, including the larynx, the lymph nodes, the pleura, the brain, the kidneys, or the bones and joints. In HIV-infected persons, extrapulmonary TB disease is often accompanied by pulmonary TB. Persons with extrapulmonary TB disease usually are not infectious unless they have 1) pulmonary disease in addition to extrapulmonary disease; 2) extrapulmonary disease located in the oral cavity or the larynx; or 3) extrapulmonary disease that includes an open abscess or lesion in which the concentration of organisms is high, especially if drainage from the abscess or lesion is extensive, or if drainage fluid is aerosolized. Persons with TB pleural effusions may have underlying pulmonary TB that is masked on chest radiograph because the effusion fluid compresses the lung. These patients should be considered infectious until pulmonary TB disease is excluded.
What is the classification system for TB?
The current clinical classification system for TB used in the United States is based on the pathogenesis of the disease (Table 2.8). It is intended mainly as an operational framework for public health programs. This classification system provides clinicians the opportunity to track the development of TB in their patients. Health-care providers should comply with state and local laws and regulations requiring the reporting of TB disease. All persons with Class 3 (clinically active) or Class 5 (TB suspected) TB should be reported promptly to the local or state health department. A patient should not have a Class 5 classification for more than 3 months.
How many people with TB will develop TB?
Without treatment, approximately 5% of persons who have been infected with M. tuberculosis will develop disease in the first year or 2 after infection, and another 5% will develop disease sometime later in life. Thus, without treatment, approximately 10% of persons with normal immune systems who are infected with M. tuberculosis will develop TB disease at some point in their lives.
How does TB spread?
A small number may multiply intracellularly and are released when the macrophages die. If alive, these bacilli may spread by way of lymphatic channels or through the bloodstream to more distant tissues and organs (including areas of the body in which TB disease is most likely to develop: regional lymph nodes, apex of the lung, kidneys, brain, and bone). This process of dissemination primes the immune system for a systemic response. Further details about pathogenesis of latent tuberculosis infection (LTBI) and TB disease are described in Figure 2.3.
How is M. tuberculosis transmitted?
Infectious droplet nuclei are generated when persons who have pulmonary or laryngeal TB disease cough, sneeze, shout, or sing. Depending on the environment, these tiny particles can remain suspended in the air for several hours. M. tuberculosis is transmitted through the air , not by surface contact. Transmission occurs when a person inhales droplet nuclei containing M. tuberculosis, and the droplet nuclei traverse the mouth or nasal passages, upper respiratory tract, and bronchi to reach the alveoli of the lungs (Figure 2.2).
Where do tuberculosis bacilli spread?
A small number of tubercle bacilli enter the bloodstream and spread throughout the body. The tubercle bacilli may reach any part of the body,including areas where TB disease is more likely to develop (such as the brain, larynx, lymph node, lung, spine, bone, or kidney).
What is the inflammatory response of M. tuberculosis?
Inflammation is a keyword here, since the growth of M. tuberculosiselicits inflammatory host responses that are necessary to control infections but can also cause extensive tissue damage. Among the cellular agents involved in tissue destruction are various proteases like cathepsin D (196) that are also thought to be major factors in the liquefaction of granulomas (58). In addition, M. tuberculosisuptake can cause apoptosis of macrophages (153, 157), and this could play a role in adjacent tissue damage. A key cytokine in the inflammatory or Th1 response of the cellular immune system is tumor necrosis factor α (TNF-α), which is necessary to control infection. Mice that are unable to produce or respond to TNF-α cannot form granulomas to restrict bacterial dissemination (260). However, when this cytokine is present in large amounts during an aerosol model of mouse infection, severe lung inflammation and early death occurs (21). TNF-α is a major determinant of disease in a rabbit model of TB meningitis, since there is a direct correlation between the extent of disease caused by several M. bovisand M. tuberculosisstrains and levels of this cytokine in the cerebrospinal fluid (291). However, data from analyses of cytokine responses and virulence in mice infected with various M. tuberculosisstrains indicate that there are factors additional to TNF-α in TB progression. M. tuberculosisCDC1551 is a clinical strain that was originally thought to be highly virulent (142); it has more recently been shown that CDC1551 induces levels of cytokines, including TNF-α, that are higher than those induced by other M. tuberculosisstrains in mice. However, it is not more virulent than the other strains, as defined by bacterial load and mortality (177). Similar results were obtained when the virulence of CDC1551 and H37Rv were compared in a rabbit model of infection (29). Also, another study compared the ability of two clinical M. tuberculosisstrains, HN878 and NHN5, to cause disease and to elicit a cytokine response in a mouse model. HN878, the more virulent of the two as determined by mortality measurements, induced smaller amounts of TNF-α and other inflammatory cytokines than NHN5 did (178). Interestingly, HN878 induced higher levels of alpha interferon (IFN-α) and Th2 cytokines like interleukin-4 (IL-4).
How does TB affect the body?
Tuberculosis (TB), one of the oldest known human diseases. is still is one of the major causes of mortality, since two million people die each year from this malady. TB has many manifestations, affecting bone, the central nervous system, and many other organ systems, but it is primarily a pulmonary disease that is initiated by the deposition of Mycobacterium tuberculosis, contained in aerosol droplets, onto lung alveolar surfaces. From this point, the progression of the disease can have several outcomes, determined largely by the response of the host immune system. The efficacy of this response is affected by intrinsic factors such as the genetics of the immune system as well as extrinsic factors, e.g., insults to the immune system and the nutritional and physiological state of the host. In addition, the pathogen may play a role in disease progression since some M. tuberculosis strains are reportedly more virulent than others, as defined by increased transmissibility as well as being associated with higher morbidity and mortality in infected individuals. Despite the widespread use of an attenuated live vaccine and several antibiotics, there is more TB than ever before, requiring new vaccines and drugs and more specific and rapid diagnostics. Researchers are utilizing information obtained from the complete sequence of the M. tuberculosis genome and from new genetic and physiological methods to identify targets in M. tuberculosis that will aid in the development of these sorely needed antitubercular agents.
Why is genetics of M. tuberculosis neglected?
Until quite recently, the genetics of M. tuberculosiswas a neglected subject because of difficulties in working with the organism and lack of suitable tools. A review published as recently as 1994 stated that this field “… is still in its infancy” (55), but the study of mycobacterial genetics has blossomed in recent years, as demonstrated by the publication an entire book dedicated to this topic (122). This is due to the development of many genetic methods, mainly by the Gicquel and Jacobs laboratories (109, 218), and to the DNA sequencing and annotation of the M. tuberculosisH37Rv genome (53) and those of related mycobacteria that have been or are currently being completed by The Institute for Genomic Research (94) and by the Sanger Center-Pasteur Institute consortium.
How many genes are in the M. tuberculosis genome?
The M. tuberculosisH37Rv genome consists of 4.4 × 106bp and contains approximately 4,000 genes (Fig. (Fig.1)1) (53). Annotation of the M. tuberculosisgenome shows that this bacterium has some unique features. Over 200 genes are annotated as encoding enzymes for the metabolism of fatty acids, comprising 6% of the total (Table (Table1).1). Among these are approximately 100 that are predicted to function in the β-oxidation of fatty acids, while E. colionly has 50 enzymes involved in fatty acid metabolism. The distantly related actinomycete Streptomyces coelicolorhas a total of 115, corresponding to a little more than 1% of the proteins, of which 59 are annotated as being involved in fatty acid degradation (23). This large number of M. tuberculosisenzymes that putatively use fatty acids may be related to the ability of this pathogen to grow in the tissues of the infected host, where fatty acids maybe the major carbon source. This important aspect of M. tuberculosisphysiology during infection is described later in this review.
How does TB cause death?
Uncontrolled M. tuberculosisgrowth in its human host, given the usual site of the infection, is associated with extensive lung damage that ultimately causes death by suffocation due to insufficient oxygen. This anoxia is caused the obliteration of lung parenchymal cells involved in oxygen uptake as well as obstruction of bronchiolar passages by granulomatous growths and by blood released during the rupture of liquefied granulomas in adjacent lung tissue (104). Other untreated forms of tuberculosis such as tubercular meningitis, which occurs in the meningial membranes of the brain, can result in death because of inflammation in brain tissue and the resulting hydrocephalus and seizures. Tuberculomas, another form of TB in the brain, are large structures formed by by the enlargement of brain granulomas, also due to inflammatory responses, and they are also associated with seizures (329). Inflammatory responses are also thought to play a role in other extrapulmonary manifestations of TB, e.g., in bone (189).
How did TB decrease in the 20th century?
TB morbidity and mortality rates due to TB steadily dropped during the 20th century in the developed world, aided by better public health practices and widespread use of the M. bovisBCG vaccine (discussed below), as well as the development of antibiotics in the 1950s. This downward trend ended and the numbers of new cases started increasing in the mid-1980s. The major causes of this were increased homelessness and poverty in the developed world and the emergence of AIDS, with its destruction of the cell-mediated immune response in coinfected persons. Only by massive expenditures of funds and human resources, mainly by directly monitored antibiotic delivery, has this “miniepidemic” of new TB cases been reversed in Europe and the United States (99).
How many people died from TB in 1800?
European immigrants to the New World brought the disease with them, and while the mortality rate never reached the levels found in Europe, large urban centers like Boston and New York had TB death rates of 6 to 7 per 1,000 in 1800, declining to 4 per 1,000 in 1860 to 1870 (62). Presumably public health measures also played a role in these declining mortality rates.
