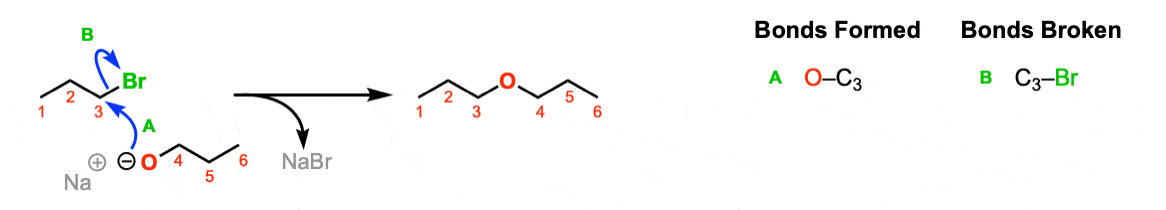
The S N 2 reaction mechanism involves the nucleophilic substitution reaction of the leaving group (which generally consists of halide groups or other electron-withdrawing groups) with a nucleophile in a given organic compound.
What is an SN2 reaction?
The SN2 reaction is a type of reaction mechanism that is common in organic chemistry. In this mechanism, one bond is broken and one bond is formed synchronously, i.e., in one step.
What is the difference between SN1 and SN2?
SN1 reactions are nucleophilic substitutions, involving a nucleophile replacing a leaving group (just like SN2). However: SN1 reactions are unimolecular:the rate of this reaction depends only on the concentration of one reactant. ●SN1 reactions happen in two steps:
What happens when S N 2 reacts with anion?
A common side reaction taking place with S N 2 reactions is E2 elimination: the incoming anion can act as a base rather than as a nucleophile, abstracting a proton and leading to formation of the alkene. This pathway is favored with sterically hindered nucleophiles.
What is the first step of the SN1 reaction?
First step of the SN1 reaction: The leaving group leaves, and the substrate carbon now only has three substituents, taking on a positive charge. This is called a carbocation. Carbocations are most stable when there are more atoms to distribute this positive charge. Carbocation stability: 3º > 2º >> 1º
Is limonene soluble in liquid CO2?
Limonene is nonpolar, therefore I would expect it to be soluble in a nonpolar solvent, like liquid CO2 or hexane.
What would happen to the overall reaction rate if the sulfuric acid was left out?
What would happen to the overall reaction rate if the sulfuric acid was left out? If the sulfuric acid was left out then the reaction would not continue.
What are the 4 factors that affect the rate of reaction?
The rate of a chemical reaction is influenced by many different factors, including reactant concentration, surface area, temperature, and catalysts.
What are the 5 factors that affect the rate of reaction?
We can identify five factors that affect the rates of chemical reactions: the chemical nature of the reacting substances, the state of subdivision (one large lump versus many small particles) of the reactants, the temperature of the reactants, the concentration of the reactants, and the presence of a catalyst.
How does the concentration of sulfuric acid affect rate of reaction?
Concentration Effects Consequently, the reaction rate usually increases as the concentration of the reactants increases. One example of this effect is the reaction of sucrose (table sugar) with sulfuric acid, which is shown in Figure 14.1.
How long does sulphuric acid take to burn skin?
Results: Damage induced by concentrated sulphuric acid appears within the first minute. Full skin lesions are observed after approximately 4 hours. No spontaneous healing of the H₂SO4 burn was observed after 2, 6 and 11 days.
Can dilute sulfuric acid burn you?
Hazard statements : May be corrosive to metals. Causes severe skin burns and eye damage.
Does chloroform react with sulfuric acid?
With sulphuric acid alone the chloroform layer is cherry red and the acid layer shows green fluorescence. With the selenious-sulphuric acid mixture, the chloroform layer assumes a deep beautiful purple, while the lower layer is red brown but does not display green fluorescence.
What is the mechanism of SN2?
In this mechanism, one bond is broken and one bond is formed synchronously, i.e., in one step. S N 2 is a kind of nucleophilic substitution reaction mechanism , the name referring to the Hughes-Ingold symbol of the mechanism. Since two reacting species are involved in the slow ( rate-determining) step, this leads to the term s ubstitution n ucleophilic ( bi -molecular) or SN2; the other major kind is S N 1. Many other more specialized mechanisms describe substitution reactions.
What is the side reaction of S N 2?
A common side reaction taking place with S N 2 reactions is E2 elimination: the incoming anion can act as a base rather than as a nucleophile, abstracting a proton and leading to formation of the alkene. This pathway is favored with sterically hindered nucleophiles. Elimination reactions are usually favoured at elevated temperatures because of increased entropy. This effect can be demonstrated in the gas-phase reaction between a sulfonate and a simple alkyl bromide taking place inside a mass spectrometer:
What are some examples of secondary substrates?
The examples in textbooks of secondary substrates going by the S N 1 mechanism invariably involve the use of bromide (or other good nucleophile) as the leaving group have confused the understanding of alkyl nucleophilic substitution reactions at secondary carbons for 80 years [3]. Work with the 2-adamantyl system (S N 2 not possible) by Schleyer and co-workers, the use of azide (an excellent nucleophile but very poor leaving group) by Weiner and Sneen, the development of sulfonate leaving groups (non-nucleophilic good leaving groups), and the demonstration of significant experimental problems in the initial claim of an S N 1 mechanism in the solvolysis of optically active 2-bromooctane by Hughes et al. [3] have demonstrated conclusively that secondary substrates go exclusively (except in unusual but predictable cases) by the S N 2 mechanism.
How does the rate of reaction affect the solvent?
The solvent affects the rate of reaction because solvents may or may not surround a nucleophile, thus hindering or not hindering its approach to the carbon atom. Polar aprotic solvents, like tetrahydrofuran, are better solvents for this reaction than polar protic solvents because polar protic solvents will hydrogen bond to the nucleophile, hindering it from attacking the carbon with the leaving group. A polar aprotic solvent with low dielectric constant or a hindered dipole end will favour S N 2 manner of nucleophilic substitution reaction. Examples: dimethylsulfoxide, dimethylformamide, acetone, etc.
What is the difference between S N 1 and S N 2?
In the S N 1 reaction the nucleophile attacks after the rate-limiting step is over, whereas in S N 2 the nucleophile forces off the leaving group in the limiting step. In other words, the rate of S N 1 reactions depend only on the concentration of the substrate while the S N 2 reaction rate depends on the concentration of both the substrate and nucleophile.
What is the most important part of a reaction?
Substrate . The substrate plays the most important part in determining the rate of the reaction. This is because the nucleophile attacks from the back of the substrate , thus breaking the carbon-leaving group bond and forming the carbon-nucleophile bond.
Why do tertiary substrates not participate in S N 2 reactions?
Tertiary substrates do not participate in S N 2 reactions, because of steric hindrance. Structures that can form highly stable cations by simple loss of the leaving group, for example, as a resonance-stabilized carbocation, are especially likely to react via an S N 1 pathway in competition with S N 2.
What is the boiling point of compound 1?
compound 1 is reported to have a boiling point of 81 C at 760 torr. will its boiling point be higher or lower in reno?
What is stationary phase?
stationary is the layer of silica and aluminum oxide. The mobile phase is a solvent system
Can a solvent ignite itself?
could cause solvent vapors to ignite and a solvent could ignite itself. Also many organic compounds can be destroyed by excessive heat
What is the SN1 and SN2 reaction?
SN1 and SN2 Reaction of Haloalkanes. Haloalkanes are converted into alcohols using hydroxide ion in aqueous media through S N 1 and S N 2 Reactions. Alcohols can efficiently be prepared by substitution of haloalkanes and sulfonic esters with good leaving groups.
Why do alkyl halides undergo S N 1 reaction?
In the case of alkyl halides, 3 o alkyl halides undergo S N 1 reaction very fast because of the high stability of 3 o carbocations.
Why is the bond between carbon and halogen broken?
The bond between carbon and halogen breaks due to the presence of a nucleophile and formation of carbocation takes place . It is the slowest and the reversible step as a huge amount of energy is required to break the bond. The bond is broken by solvation of the compound in a protic solvent, thus this step is slowest of all.
What happens in the first step of a carbon-halogen bond?
In the second step, the nucleophile reacts rapidly with the carbocation that was formed in the first step. This reaction is carried out in polar protic solvents such as water, alcohol, acetic acid etc.
What are the factors that affect the nucleophilic substitution reaction?
Some important factors include. Effect of the solvent. Effect of the structure of the substrate. Effect of the nucleophile. Effect of leaving-group.
Does the rate of reaction depend on haloalkane?
The rate of reaction depends only on haloalkane, not on nucleophile. The nucleophile attacks the carbocation formed in step 1 and the new compound is formed. Since, the rate defining step of the reaction is the formation of a carbocation, hence greater the stability of formation of an intermediate carbocation, more is the ease ...
Is carbocation a one step reaction?
It is a one-step reaction. Both the formation of carbocation and exiting of halogen take place simultaneously. In this process, unlike the S N 1 mechanism, the inversion of configuration is observed. Since this reaction requires the approach of the nucleophile to the carbon bearing the leaving group, the presence of bulky substituents on or near the carbon atom has a dramatic inhibiting effect.

Overview
The SN2 reaction is a type of reaction mechanism that is common in organic chemistry. In this mechanism, one bond is broken and one bond is formed synchronously, i.e., in one step. SN2 is a kind of nucleophilic substitution reaction mechanism, the name referring to the Hughes-Ingold symbol of the mechanism. Since two reacting species are involved in the slow (rate-determining) step, thi…
Reaction mechanism
The reaction most often occurs at an aliphatic sp carbon center with an electronegative, stable leaving group attached to it (often denoted X), which is frequently a halide atom. The breaking of the C–X bond and the formation of the new bond (often denoted C–Y or C–Nu) occur simultaneously through a transition state in which a carbon under nucleophilic attack is pentacoordinate, …
Factors affecting the rate of the reaction
Four factors affect the rate of the reaction:
The substrate plays the most important part in determining the rate of the reaction. This is because the nucleophile attacks from the back of the substrate, thus breaking the carbon-leaving group bond and forming the carbon-nucleophile bond. Therefore, to maximise the rate of the SN2 reaction, the back of the substrate must be as unhindered as possible. Overall, this means that …
Reaction kinetics
The rate of an SN2 reaction is second order, as the rate-determining step depends on the nucleophile concentration, [Nu ] as well as the concentration of substrate, [RX].
r = k[RX][Nu ]
This is a key difference between the SN1 and SN2 mechanisms. In the SN1 reaction the nucleophile attacks after the rate-limiting step is over, whereas in SN2 the nucleophile forces off …
E2 competition
A common side reaction taking place with SN2 reactions is E2 elimination: the incoming anion can act as a base rather than as a nucleophile, abstracting a proton and leading to formation of the alkene. This pathway is favored with sterically hindered nucleophiles. Elimination reactions are usually favoured at elevated temperatures because of increased entropy. This effect can be demo…
Roundabout mechanism
A development attracting attention in 2008 concerns a SN2 roundabout mechanism observed in a gas-phase reaction between chloride ions and methyl iodide with a special technique called crossed molecular beam imaging. When the chloride ions have sufficient velocity, the initial collision of it with the methyl iodide molecule causes the methyl iodide to spin around once before the actual SN2 displacement mechanism takes place.
See also
• Arrow pushing
• Christopher Kelk Ingold
• Finkelstein reaction
• Neighbouring group participation
• Nucleophilic acyl substitution
What Is SN2 Reaction Mechanism?
Table of Contents
What Is An SN2 reaction?
- The SN2 reaction is a nucleophilic substitution reaction where a bond is broken and another is formed synchronously. Two reacting species are involved in the rate determining step of the reaction. The term ‘SN2’ stands for – Substitution Nucleophilic Bimolecular. This type of reaction is also referred to as bimolecular nucleophilic substitution, associative substitution, and intercha…
SN2 Reaction Mechanism
- This reaction proceeds through a backside attack by the nucleophile on the substrate. The nucleophile approaches the given substrate at an angle of 180o to the carbon-leaving group bond. The carbon-nucleophile bond forms and carbon-leaving group bond breaks simultaneously through a transition state. Now, the leaving group is pushed out of the trans...
Stereochemistry of SN2 Reactions
- There are two ways in which the nucleophile can attack the stereocenter of the substrate: 1. A frontside attack where the nucleophile attacks from the same side where the leaving group is present, resulting in the retention of stereochemical configurationin the product. 2. A backside attackwhere the nucleophile attacks the stereocenter from the opposite side of the carbon-leavin…