
Physical Properties:
- Fats and fatty acids are soluble in organic solvents, such as petroleum ether, benzene and chloroform. They are insoluble in water. ...
- Saturated fatty acids are solid at room temperature, while unsaturated fatty acids are liquid.
- Unsaturated fatty acids show cis-trans isomerism due to presence of double bonds.
- They are bad conductors of heat.
- The crystallization.
- The melting point.
- The viscosity.
- The refractive index.
- The density.
- The solubility.
- The plasticity.
- The emulsifying capacity.
What are the physical characteristics of fat?
Fats are made up of a combination of different fatty acids, but one type generally predominates, which determines the physical characteristics. Fats that contain a high proportion of SFA, such as butter or lard, are solid at room temperature and have a relatively high melting temperature.
What are the physical properties of oils and fats?
Physical properties of oils and fats 1 Crystallization. Fats differ from oils in their degree of solidification at room temperature, since in these conditions the oils are in a liquid state (not crystallized) while the fats are ... 2 Melting point. ... 3 Viscosity. ... 4 Refractive index. ...
What is Fatfat and how does it work?
Fat is the most concentrated source providing 9 kcal per 1 gram consumed, which is more than double the energy content of protein or carbohydrate (4 kcal per gram) and more than quadruple the energy content of fibre (2 kcal per gram). Fat can be stored in the body’s fat tissue, which releases fatty acids when energy is required (see box: Body fat).
What is the basic chemistry of fats?
Understanding the basic chemistry of fats will help to understand the role that fats play in our health and in food technology. Over 90% of dietary fats are in the form of triglycerides, which consist of a glycerol backbone with fatty acids esterified on each of the three hydroxyl groups of the glycerol molecule. Figure 1.
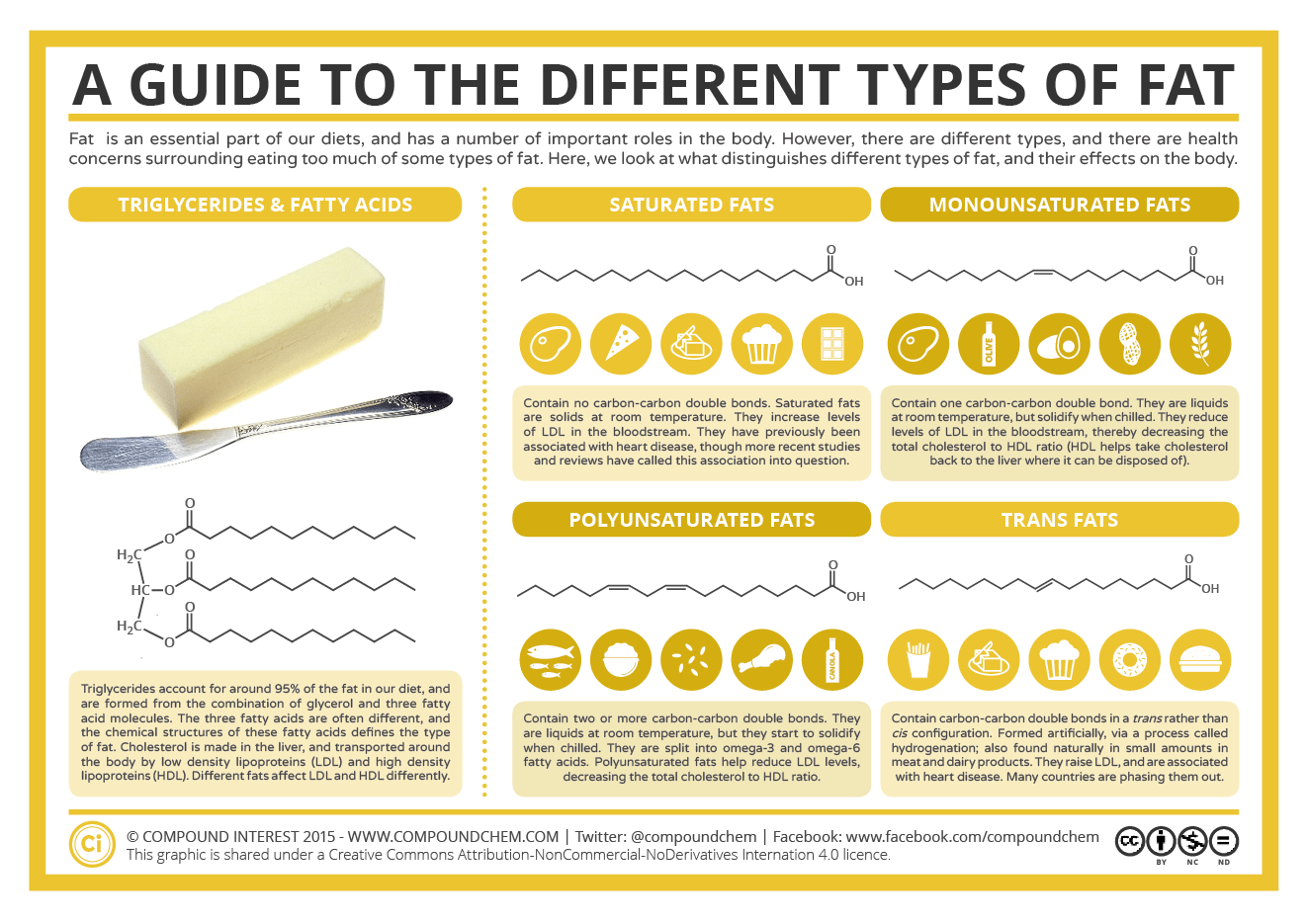
What are the functional properties of fat?
Functional properties of fats and oils are described below.Heat Transfer. Due to their low specific heat capacity and high boiling point, fats and oils can heat up quickly and reach very high temperatures. ... Flavor retention. Most flavors are hydrophobic in nature, making fats and oils a good solvent for them. ... Texture.
What are the 4 main functions of fats?
The 4 functions of fat are as follows:Fat is an important storage form of food.It produces energy in the body such as carbohydrates.Fat serves as a solvent for the fat-soluble vitamins.The fat lying underneath the skin renders protection to the body against a rapid heat loss.
What are 3 properties of lipids?
Properties of LipidsLipids may be either liquids or non-crystalline solids at room temperature.Pure fats and oils are colorless, odorless, and tasteless.They are energy-rich organic molecules.Insoluble in water.Soluble in organic solvents like alcohol, chloroform, acetone, benzene, etc.No ionic charges.More items...•
What are the 5 main functions of fat?
Types of Fat.#1 Function of Fat: Forms the Structural Component of Cells.#2 Function of Fat: Source of Energy.#3 Functions of Fat: Absorption of Fat-Soluble Vitamins.#4 Function of Fat: Insulates and Protects the Body.#5 Function of Fat: Increases Satiety.Conclusion and My Recommendations.
What are the 7 main functions of fat?
Functions of Fat in FoodAppearance.Emulsions.Flavor.Heat Transfer.Melting Point.Nutrition.Satiety.Shortening.More items...•
What is the importance of fats?
Fat is a source of essential fatty acids, which the body cannot make itself. Fat helps the body absorb vitamin A, vitamin D and vitamin E. These vitamins are fat-soluble, which means they can only be absorbed with the help of fats.
What are the properties of protein?
Physical Properties of ProteinsColour and Taste. Proteins are colourless and usually tasteless. ... Shape and Size. The proteins range in shape from simple crystalloid spherical structures to long fibrillar structures. ... Molecular Weight. ... Colloidal Nature. ... Denaturation. ... Amphoteric Nature. ... Ion Binding Capacity. ... Solubility.More items...
What are the chemical properties of fatty acids?
A fatty acid is composed of a straight chain of an even number of carbon atoms, with hydrogen atoms along the length of the chain and at one end, and a carboxyl group (COOH) at the other end. It is the carboxyl group that causes it to be an acid (carboxylic acid).
Which of the following is a property of lipid?
Lipids are compounds of carbon, hydrogen and oxygen where ratio of hydrogen and oxygen is less than that of water. Lipids are esters of fatty acids and alcohol which form emulsion with water but are soluble in organic solvents.
What are the 5 different types of fat?
Types of FatsSaturated fat. Saturated fat is solid at room temperature, which is why it is also known as "solid fat." It is mostly in animal foods, such as milk, cheese, and meat. ... Trans fat. This is a fat that has been changed by a process called hydrogenation. ... Unsaturated fat. ... Total fat.
What is fat?
Fat is defined as a substance made up of carbon, hydrogen and oxygen, not soluble in water.
What are fats good for? Functions of fats
The body needs fat to work properly. The main functions of fat are the following:
What is the chemical property of fats?
Their main chemical property is the reaction with water (hydrolysis). It easily occurs in the presence of catalysts - alkali, oxides of magnesium, zinc or calcium. A mixture of carboxylic acids and glycerin is found in the reaction products. Since the reaction of fats with water is reversible, the industry creates conditions under which it passes ...
Why do we have fat in our cells?
Due to metabolic reactions, fats in the cells of the body can be synthesized from an excess of carbohydrates. This explains the fact that uncontrolled consumption of foods rich in starch and sucrose (flour products, rice, potatoes, sweets), leads to excess weight.
How are vegetable fats formed?
They contain molecules of unsaturated carboxylic acids having double bonds. Vegetable fats are synthesized in the channels of the endoplasmic reticulum under the action of enzymes from glycerol and fatty acids. And they, in turn, are formed in the reactions of the Calvin cycle, occurring as a result of photosynthesis. Drops of oil accumulate in seeds, fruits, less often in the vegetative parts of plants and serve as a supply of nutrients. The physicochemical properties of the fats produced by plants are due to the presence of a double pi bond in their molecules. At the point of its rupture, addition reactions occur, for example, of hydrogen atoms. This leads to the formation of solid hydrogenated triglycerides.
What are the physical properties of triglycerides?
Physical properties. Both natural and synthetic, for example, margarine, triglycerides have common features. The main one is hydrophobicity, low melting point and low specific gravity. They are well soluble in organic solvents, for example, in benzene, carbon tetrachloride.
Where is fat deposited in the body?
In the villi of the small intestine, fat molecules synthesized from them are characteristic of the human body, and then they are absorbed into the lymph. Through the lymphatic vessels, fats enter the cells, and their excess is deposited in the subcutaneous fatty tissue or omentum.
Which organ is located in the fat layer?
Such vital organs as the kidneys must be located in the fat layer. With a sharp loss of weight in humans due to the thinning of this layer, kidne y prolapse can be observed, which is a serious pathology that disrupts the work of the excretory system. The importance of lipids in the formation of cell membranes.
Is triglyceride a fat?
As it was proved by the experiments of M. Berthelot and E. Chevrel, triglycerides are esters of trihydric alcohol glycerol and higher monobasic carboxylic acids. Fat containing steoric or palmetic acid is hard, for example, beef, pork, mutton. These are animal fats.
What are the physical properties of oils and fats?
For this, the crystallization, the melting point, the viscosity, the refractive index, the density, the solubility, ...
How do fats differ from oils?
Fats differ from oils in their degree of solidification at room temperature, since in these conditions the oils are in a liquid state (not crystallized) while the fats are in the solid (crystallized) state . The proportion of crystals in fats have great importance in determining the physical properties of a product.
How big are fat crystals?
The fat crystals have a size between 0.1 and 0.5 μm and can occasionally reach up to 100 μm. The crystals are maintained by Van der Waalls forces forming a three-dimensional network that provides rigidity to the product.
Why is refractive index important?
This physical property is of great importance when it comes to designing equipment to process grease. When the fats melt, their volume increases and therefore the density decreases.
How does fat become plastic?
The plasticity of a fat is caused by the presence of a three-dimensional network of crystals inside which liquid fat is immobilized. For a grease to be plastic and extensible there must be a ratio between the solid and liquid part (20 -40% solid state fat), the nets must not be tight and their crystals must be in α form.
Why do phospholipids interact with water?
Phospholipids can interact with water because the phosphoric acid and the alcohols that compose them have hydrophilic groups.
Why is the viscosity of fat so high?
The viscosity of a fat is due to the internal friction between the lipids that constitute it. It is generally high due to the high number of molecules that make up a fat.
What are the functions of fats?
Dietary fats are not just a source of energy; they function as structural building blocks of the body, carry fat-soluble vitamins, are involved in vital physiological processes in the body, and are indispensable for a number of important biological functions including growth and development. The importance of dietary fats is explained in more detail below.
Why are fats important?
Dietary fats are not just a source of energy; they function as structural building blocks of the body , carry fat-soluble vitamins, are involved in vital physiological processes in the body, and are indispensable for a number of important biological functions including growth and development. The importance of dietary fats is explained in more ...
How much cholesterol is in the small intestine?
The amount of cholesterol, which passes daily through the small intestine, which is the sum of dietary cholesterol and produced cholesterol, is between 1 and 2 g. 2 The average cholesterol intake in Europe is 200-300 mg/day, meaning that the body’s production is significantly higher.
What are the structural components of the cell?
Structural component. The membranes around the cells in our body physically separate the inside from the outside of the cell, and control the movement of substances in and out of the cells. They are mainly made of phospholipids, triglycerides and cholesterol (see Functions, Classification and Characteristics of Fats ).
What is the brain's fatty acid composition?
1. The brain is very rich in fat (60%) and has a unique fatty acid composition; docosahexaenoic acid (DHA) is the major brain fatty acid. The lipids of the retina also contain very high ...
How do length and saturation affect the arrangement of the membrane?
Both length and saturation of the fatty acids from phospholipids and triglycerides affect the arrangement of the membrane and thereby its fluidity. Shorter chain fatty acids and unsaturated fatty acids are less stiff and less viscous, making the membranes more flexible.
What is the carrier of vitamins?
Carrier of vitamins. In the diet, fat is a carrier for the fat-soluble vitamin s A, D, E and K, and supports their absorption in the intestine. Consuming sufficient amounts of fatty foods that contain these vitamins is thus essential for adequate intake of these micronutrients.
History of the discovery
The structure was studied in the middle of the 19th century. The French chemist E. Shevrel heated them with water in the presence of alkali and found in the reaction products molecules of fatty carboxylic acids and glycerol. M.
Fats - esters
As it was proved by the experiments of M. Bertlo and E. Chevrel, triglycerides are esters of the triatomic alcohol of glycerin and higher monobasic carboxylic acids. Fat containing steroinic or palmitic acid is solid, for example, beef, pork, lamb. These are animal fats.
Physical properties
Both natural and synthetic, for example, margarine, triglycerides have common characteristics. The main one is hydrophobicity, low melting point and low specific gravity. They are readily soluble in organic solvents, for example, in benzene, carbon tetrachloride. All fats are easily absorbed by porous or fibrous materials.
Chemical reactions of triglycerides
The quantitative and qualitative composition of the fat molecule, as well as its spatial configuration, confirms the fact that triglycerides belong to the class of esters. Their main chemical property is the reaction with water (hydrolysis). It easily occurs in the presence of catalysts - alkalis, magnesium oxides, zinc or calcium.
Reaction of alkaline saponification
Continue to study organic substances - esters. Fats, whose chemical properties are represented by the hydrolysis reaction, are also able to react with alkalis. This reaction is called saponification and it is the opposite of the esterification process.
Liquid Fats - Oils
They include molecules of unsaturated carboxylic acids having double bonds. Vegetable fats are synthesized in the channels of the endoplasmic reticulum under the action of enzymes from glycerin and fatty acids. And they, in turn, are formed in the reactions of the Calvin cycle, resulting from photosynthesis.
Chemical properties of vegetable fats
As mentioned earlier, triglycerides of plant origin contain higher unsaturated carboxylic acids. Oils can be processed by hydrogenation. This process is carried out with heating and in the presence of a catalyst - powdered nickel.
Which type of fat is found in high concentrations?
1. Monounsaturated fats are found in high concentrations in: 2. Polyunsaturated fats are found in high concentrations in. Canola oil – though higher in monounsaturated fat, it’s also a good source of polyunsaturated fat. Omega-3 fats are an important type of polyunsaturated fat.
What are the benefits of unsaturated fats?
Unsaturated fats, which are liquid at room temperature, are considered beneficial fats because they can improve blood cholesterol levels, ease inflammation, stabilize heart rhythms, and play a number of other beneficial roles . Unsaturated fats are predominantly found in foods from plants, such as vegetable oils, nuts, and seeds.
Why are trans fats bad for you?
Trans fats are the worst type of fat for the heart, blood vessels, and rest of the body because they: Raise bad LDL and lower good HDL. Create inflammation, ( 18) – a reaction related to immunity – which has been implicated in heart disease, stroke, diabetes, and other chronic conditions.
What is saturated fat?
Saturated Fats. All foods containing fat have a mix of specific types of fats. Even healthy foods like chicken and nuts have small amounts of saturated fat, though much less than the amounts found in beef, cheese, and ice cream. Saturated fat is mainly found in animal foods, but a few plant foods are also high in saturated fats, such as coconut, ...
How to get Omega 3?
An excellent way to get omega-3 fats is by eating fish 2-3 times a week. Good plant sources of omega-3 fats include flax seeds, walnuts, and canola or soybean oil. Higher blood omega-3 fats are associated with lower risk of premature death among older adults, according to a study by HSPH faculty.
Is canola oil a good source of polyunsaturated fat?
Canola oil – though higher in monounsaturated fat, it’s also a good source of polyunsaturated fat. Omega-3 fats are an important type of polyunsaturated fat. The body can’t make these, so they must come from food. An excellent way to get omega-3 fats is by eating fish 2-3 times a week.
Does a monounsaturated diet lower blood pressure?
More recently, a randomized trial known as the Optimal Macronutrient Intake Trial for Heart Health (OmniHeart) showed that replacing a carbohydrate-rich diet with one rich in unsaturated fat, predominantly monounsaturated fats, lowers blood pressure, improves lipid levels, and reduces the estimated cardiovascular risk.

What Are Dietary Fats?
Zooming in on The Molecular Structure, How Are Dietary Fats built?
- Understanding the basic chemistry of fats will help to understand the role that fats play in our health and in food technology. Over 90% of dietary fats are in the form of triglycerides, which consist of a glycerol backbone with fatty acidsesterified on each of the three hydroxyl groups of the glycerol molecule. Figure 1. Structure of a triglyceride and saturated, monounsaturated and …
Summary
- Dietary fats are an important part of our diet, delivering about 20-35% of our daily energy needs. Beyond energy, they are indispensable for a number of important biological functions including growth and development. This first part of the EUFIC review Facts on Fats - the Basics, explains what dietary fats actually are, where they can be found, what is their molecular structure, and wh…
Annex 1. List of Most Common Fatty Acids
- (*) The figure before the colon indicates the number of carbon atoms the fatty acid molecule contains, and the figure after the colon indicates the total number of double bonds. The n-(omega) designation gives the position of the first double bond counting from the methyl end of the fatty acid molecule.
References
- Brouwer I, Wanders A & Katan M (2013). Trans fatty acids and cardiovascular health: research completed? European Journal of Clinical Nutrition 67(5): 1-7.
- Brenna T, Salem N, Sinclair A, et al. (2009). α-Linolenic Acid Supplementation and Conversion to n-3 Long Chain PUFA in Humans.
- Commission of The European Communities (2007). White Paper On A Strategy for Europe o…
- Brouwer I, Wanders A & Katan M (2013). Trans fatty acids and cardiovascular health: research completed? European Journal of Clinical Nutrition 67(5): 1-7.
- Brenna T, Salem N, Sinclair A, et al. (2009). α-Linolenic Acid Supplementation and Conversion to n-3 Long Chain PUFA in Humans.
- Commission of The European Communities (2007). White Paper On A Strategy for Europe on Nutrition, Overweight and Obesity related health issues. Brussels, Belgium.
- Hayes K & the Expert Panel (2010). Fatty acid expert roundtable: key statements about fatty acids. Journal of the American College of Nutrition 29(Suppl 3): S285-S288.
Crystallization
Melting Point
Viscosity
Refractive Index
Density
Solubility
- Solubility has great relevance in the processing of fats. Fats are fully soluble apolar solvents (benzene, hexane …). Except for phospholipids, they are completely insoluble in polar solvents (water, acetonitrile). They are partially soluble in solvents of intermediate polarity (alcohol, acetone) The solubility of fats in organic solventsdecreases ...
Plasticity
Emulsifying Capacity