Summary of benzene electrophilic substitution reactions
| Name of reaction | Reactant | Catalyst | Products |
| Nitration | HNO 3 | H 2 SO 4 | C 6 H 5 NO 2, H 2 O |
| Chlorination | Cl 2 | AlCl 3 | C 6 H 5 Cl, HCl |
| Friedel-Crafts acylation with acyl chlor ... | RCOCl | AlCl 3 | C 6 H 5 COR, HCl |
| Friedel-Crafts alkylation | RX | AlCl 3 | C 6 H 5 R, HCl |
| Reaction Type | Typical Equation | |
|---|---|---|
| Halogenation: | C6H6 | C6H5Cl + HCl Chlorobenzene |
| Nitration: | C6H6 | C6H5NO2 + H2O Nitrobenzene |
| Sulfonation: | C6H6 | C6H5SO3H + H2O Benzenesulfonic acid |
| Alkylation: Friedel-Crafts | C6H6 | C6H5-R + HCl An Arene |
What drugs have benzene in them?
What drugs have benzene in them?
- Paint, lacquer, and varnish removers.
- Industrial solvents.
- Gasoline and other fuels.
- Glues.
- Paints.
- Furniture wax.
- Detergents.
- Thinners.
What is the reactivity of benzene?
The common Benzene Reactions involve halogenation, sulfonation and nitration of benzene. Benzene is an organic compound which is made up of six carbon atoms arranged in a planar ring, each containing one hydrogen atom. The molecular formula of Benzene is C 6 H 6 and was first discovered by Michael Faraday in the year 1825.
What to know about benzene and cancer amid Procter?
“Benzene is classified as a human carcinogen. Exposure to benzene can occur by inhalation, orally, and through the skin and it can result in cancers including leukemia and blood cancer of the bone marrow and blood disorders which can be life-threatening,” the release said.
What are the effects of benzene exposure?
- A) USES: Most commonly used as an intermediate in the production of other chemicals. ...
- B) TOXICOLOGY: Human metabolism of benzene to its principal metabolites (ie, hydroquinone, catechol, and phenol) occurs via cytochrome P450 enzyme 2E1 and quinone oxidoreductase NQ01. ...
- C) EPIDEMIOLOGY: Acute exposure is very rare. ...
See more

What are the main reactions of benzene?
Benzene Reactions - Sulfonation, Nitration and Halogenation.
What are the addition reactions of benzene?
Key PointsAddition ReactionBenzene +ConditionsHydrogenationH 21 5 0 ∘ C – 1 9 0 ∘ C High pressuresChlorinationC l 2UV light HeatBrominationB r 2UV light Heat
In which reaction benzene is formed?
Benzene is prepared from ethyne by the process of cyclic polymerization. In this process, Ethyne is passed through a red-hot iron tube at 873 K. The ethyne molecule then undergoes cyclic polymerization to form benzene.
What is acylation of benzene?
What is acylation? An acyl group is an alkyl group attached to a carbon-oxygen double bond. If "R" represents any alkyl group, then an acyl group has the formula RCO-. Acylation means substituting an acyl group into something - in this case, into a benzene ring. The most commonly used acyl group is CH3CO-.
Does benzene give elimination reaction?
Benzene cannot undergo Elimination reaction.
Why is benzene so reactive?
The molecular formula of benzene is C6H6. It is evident from the molecular formula that the organic compound is highly unsaturated. Due to its high degree of unsaturation, it is highly reactive.
Which reaction is not given by benzene?
Thus, benzene does not give addition reactions because of resonance stabilisation.
What is another name for benzene?
BenzolBenzeneNamesPreferred IUPAC name BenzeneOther names Benzol (historic/German) Cyclohexa-1,3,5-triene; 1,3,5-Cyclohexatriene [6]Annulene (not recommended)IdentifiersCAS Number71-43-269 more rows
Why does benzene show addition reaction?
In benzene, the π-electrons are delocalised and makes the structure more stable. Delocalization of π electron is called resonance. Thus, benzene does not give addition reactions because of resonance stabilisation.
What are addition reactions of alkenes?
The most common type of reaction for alkene is the addition reaction to a C=C double bond. In addition reaction, a small molecule is added to multiple bonds, and one π bond is converted to two σ bonds (unsaturation degree decreases) as a result of the addition.
How do you add a benzene group?
An alkyl group can be added to a benzene molecule by an electrophile aromatic substitution reaction called the Friedel‐Crafts alkylation reaction. One example is the addition of a methyl group to a benzene ring. The mechanism for this reaction begins with the generation of a methyl carbocation from methylbromide.
What is hydrogenation of benzene?
Hydrogenation. Hydrogenation is an addition reaction in which hydrogen atoms are added all the way around the benzene ring. A cycloalkane is formed.
How does benzene react?
As we explored above, benzene contains delocalised pi electrons found in a ring. This ring of delocalisation is relatively strong and stable because it spreads the charge of the electrons over a greater area. It takes a lot of energy to disrupt the delocalisation.
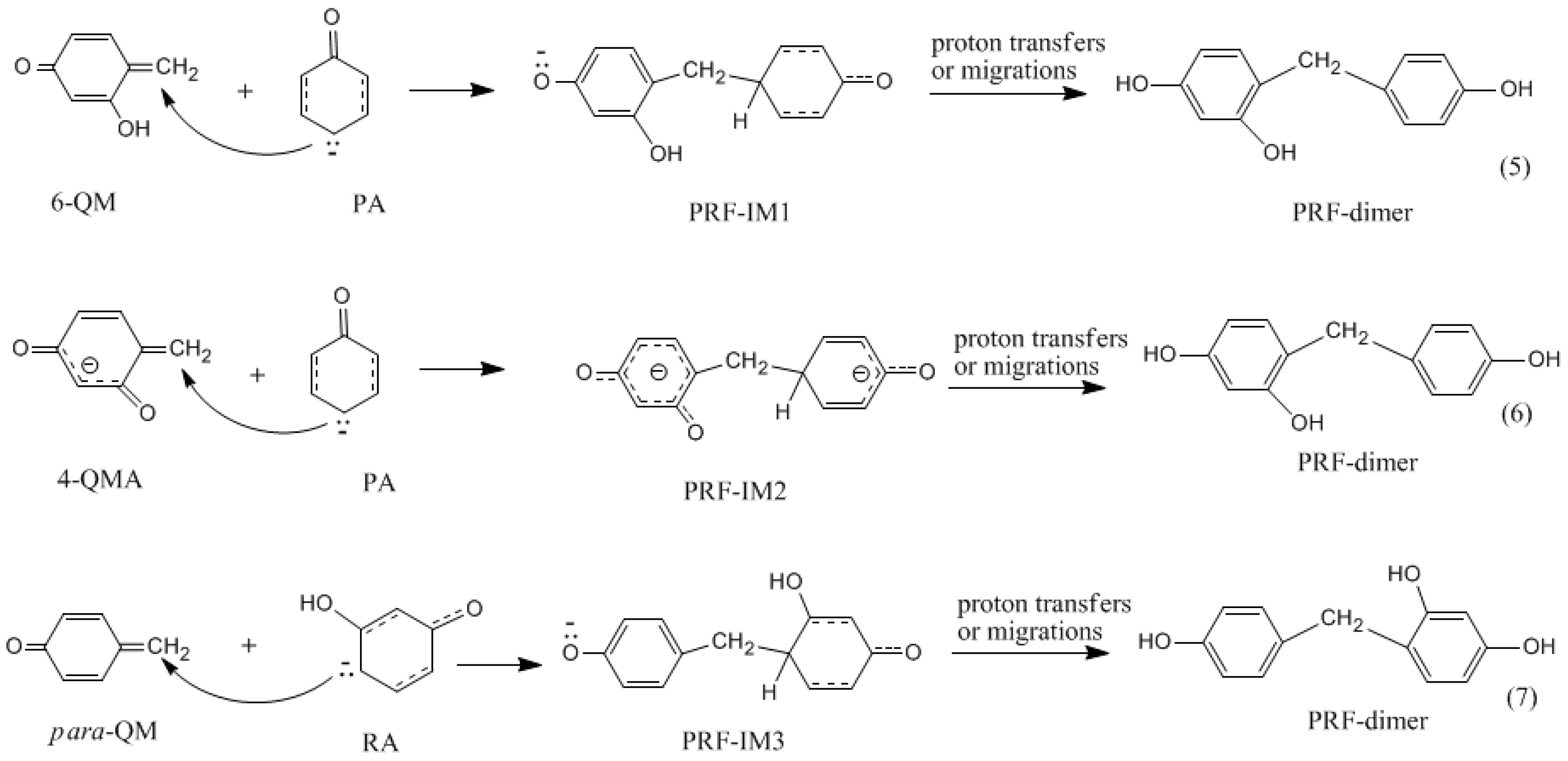
Electrophilic substitution reactions of benzene
To start, let’s first look at the general mechanism of an electrophilic substitution reaction with benzene. The electrophile is represented by .
Other reactions of benzene
Although electrophilic substitution reactions are the most common type of reaction involving benzene, aromatic compounds do take part in other reactions. You don’t need to know the mechanisms for these reactions. Some examples include:
Reactions of Benzene - Key takeaways
Benzene is an aromatic compound with the formula C6H6. It contains a ring of delocalised electrons.
Reactions of Benzene
Bromination of benzene is a type of electrophilic substitution reaction.
What is the reaction of nitration of benzene?
Nitration of Benzene. Benzene reacts with concentrated nitric acid at 323-333k in the presence of concentrated sulphuric acid to form nitrobenzene. This reaction is known as nitration of benzene. Nitration of nitrobenzene.
What is the electrophilic substitution reaction of benzene?
Generally, the electrophilic substitution reaction of benzene is a three-step process involving: Generation of the electrophile. Intermediate carbocation formation. Removal of a proton from carbocation intermediate.
What is the process of sulfonation of benzene?
Sulfonation of Benzene. Sulfonation of benzene is a process of heating benzene with fuming sulphuric acid (H2SO4 +SO3) to produce benzenesulfonic acid. The reaction is reversible in nature.
How does FeBr3 help in halogenation?
The mechanism for halogenation of benzene: Step 1: Being a Lewis acid, FeBr3 helps in the generation of electrophile bromine ion by combining with the attacking reagent. Generation of bromine ion.
Which ion is used to form bromobenzene?
Step 2: The bromine ion acts as an electrophile in the process which further reacts with benzene to form arenium ion which finally converts to bromobenzene.
Is benzene a stable element?
8,992. Benzene is highly prone to electrophilic substitution reactions compared to addition reactions as it loses its aromaticity during addition reaction. As benzene contains delocalized electrons spanning over carbon atoms in the ring, it is highly attractive to electrophiles and is also highly stable to electrophilic substitutions.
Benzene
Benzene is classified as a hydrocarbon since it solely has carbon and hydrogen atoms.
Structure of Benzene
A six-carbon ring (shown by a hexagon) with three double bonds is the most common structural depiction for benzene.
Reactions of Benzene
Electrophilic substitution reactions are more common in benzene than addition reactions because it loses its aromaticity during addition reactions.
Nitration of Benzene
In the nitration of benzene, concentrated nitric acid and concentrated sulfuric acid are used to treat benzene at a temperature not exceeding 50°C.
Sulfonation of Benzene
The electrophilic substitution reaction between benzene and sulfuric acid is known as the sulfonation of benzene.
Alkylation and Acylation of Benzene
Alkylbenzenes are produced through the Friedel-Crafts Alkylation process from alkyl halides.
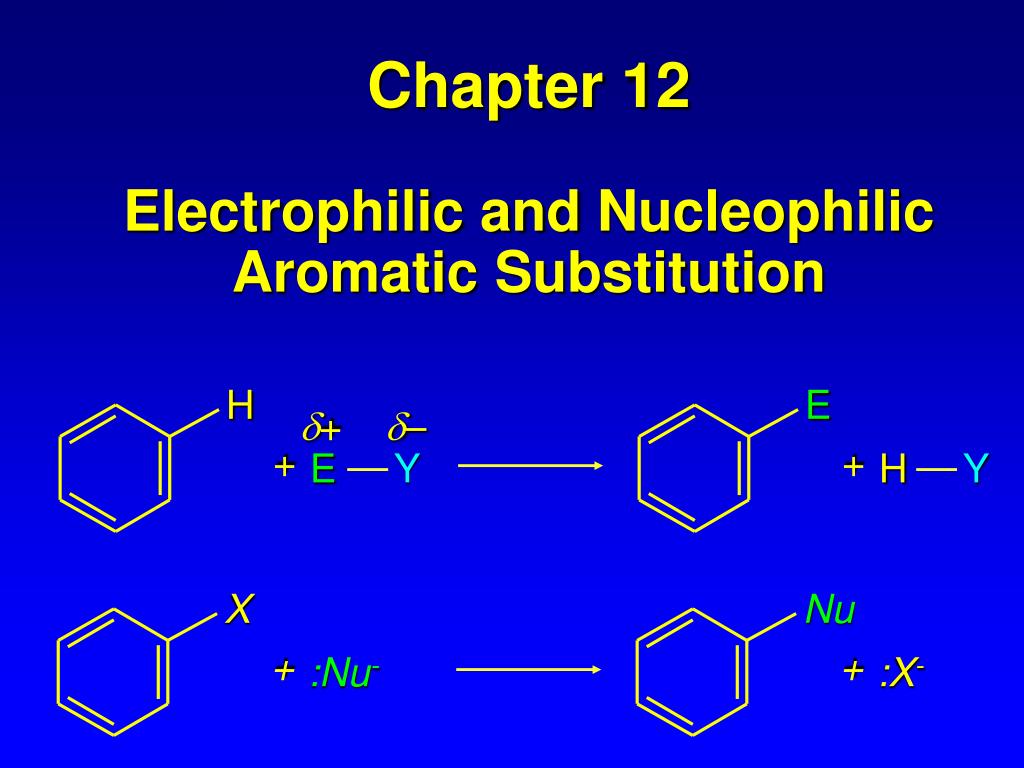
Nucleophilic Aromatic Substitution
A nucleophilic aromatic substitution occurs when a strong leaving group, such as a halide, is relocated on an aromatic ring by the nucleophile. This response is mostly governed by addition-elimination reaction mechanisms.
Definition: Substitution Reaction
A substitution reaction is a type of reaction where parts of a molecule are removed and replaced with other functional groups.
Definition: Functional Group
A functional group is a portion of a molecule with a particular composition and arrangement; functional groups usually behave in particular, predictable ways.
Definition: Halogen Substitution Reaction
A halogen substitution reaction is a type of reaction where parts of a molecule are removed and replaced with halogens.
Definition: Alkyl Substitution Reaction
An alkyl substitution reaction is a type of reaction where parts of a molecule are removed and replaced with alkyl groups.
Example 1: Predicting the Product That Would Form from the Alkylation of Benzene given the Reaction Equation
Express the product that will be formed from the alkylation of benzene as shown in the reaction scheme.
Answer
In the reaction scheme, we can see benzene ( C H 6 6) reacting with 2-chloro-2-methylpropane. There is also a reagent above the reaction arrow: aluminum chloride, A l C l 3. This is a classic example of a Friedel–Crafts alkylation.
Definition: Nitro Substitution Reaction
A nitro substitution reaction is a type of reaction where parts of a molecule are removed and replaced with nitro groups ( N O 2 ).
What are the symptoms of high levels of benzene?
Death (at very high levels) Eating foods or drinking beverages containing high levels of benzene can cause the following symptoms within minutes to several hours: Vomiting. Irritation of the stomach. Dizziness. Sleepiness. Convulsions. Rapid or irregular heartbeat.
How does benzene affect the body?
(Long-term exposure means exposure of a year or more.) Benzene causes harmful effects on the bone marrow and can cause a decrease in red blood cells, leading to anemia.
How does benzene affect the immune system?
For example, it can cause bone marrow not to produce enough red blood cells, which can lead to anemia. Also, it can damage the immune system by changing blood levels of antibodies and causing the loss of white blood cells.
How to get rid of benzene in your body?
Washing yourself. As quickly as possible, wash any benzene from your skin with large amounts of soap and water. Washing with soap and water will help protect people from any chemicals on their bodies. If your eyes are burning or your vision is blurred, rinse your eyes with plain water for 10 to 15 minutes.
How to protect yourself from benzene?
First, if the benzene was released into the air, get fresh air by leaving the area where the benzene was released. Moving to an area with fresh air is a good way to reduce the possibility of death from exposure to benzene in the air.
What is the source of benzene in the air?
How you could be exposed to benzene. Outdoor air contains low levels of benzene from tobacco smoke, gas stations, motor vehicle exhaust, and industrial emissions. Indoor air generally contains levels of benzene higher than those in outdoor air. The benzene in indoor air comes from products that contain benzene such as glues, paints, furniture wax, ...
How serious is benzene poisoning?
The seriousness of poisoning caused by benzene depends on the amount, route, and length of time of exposure, as well as the age and preexisting medical condition of the exposed person.
How do substituents affect reactivity?
I. The first is the relative reactivity of the compound compared with benzene itself. Experiments have shown that substituents on a benzene ring can influence reactivity in a profound manner. For example, a hydroxy or methoxy substituent increases the rate of electrophilic substitution about ten thousand fold, as illustrated by the case of anisole in the virtual demonstration (above). In contrast, a nitro substituent decreases the ring's reactivity by roughly a million. This activation or deactivation of the benzene ring toward electrophilic substitution may be correlated with the electron donating or electron withdrawing influence of the substituents, as measured by molecular dipole moments. In the following diagram we see that electron donating substituents (blue dipoles) activate the benzene ring toward electrophilic attack, and electron withdrawing substituents (red dipoles) deactivate the ring (make it less reactive to electrophilic attack).
What happens when carbocation intermediates are formed?
The cation may bond to a nucleophile to give a substitution or addition product. 2. The cation may transfer a proton to a base, giving a double bond product. 3.
Is S N 1 a mode of reaction?
S N 1 and E1 reactions are respective examples of the first two modes of reaction. The second step of alkene addition reactions proceeds by the first mode, and any of these three reactions may exhibit molecular rearrangement if an initial unstable carbocation is formed. The carbocation intermediate in electrophilic aromatic substitution (the benzenonium ion) is stabilized by charge delocalization (resonance) so it is not subject to rearrangement. In principle it could react by either mode 1 or 2, but the energetic advantage of reforming an aromatic ring leads to exclusive reaction by mode 2 ( ie. proton loss).
Is benzene an unsaturated hydrocarbon?
The remarkable stability of the unsaturated hydrocarbon benzene has been discussed in an earlier section. The chemical reactivity of benzene contrasts with that of the alkenes in that substitution reactions occur in preference to addition reactions, as illustrated in the following diagram (some comparable reactions of cyclohexene are shown in the green box).
Structure of Benzene
Applications of Nitrobenzene
- Nitrobenzene is widely used in manufacturing of the chemical, aniline. It finds its application in lubricating oils used in motors and machines. It is also used in manufacturing of dyes, drugs, pesticides, and synthetic rubber. 3. Sulfonation of Benzene Sulfonation of benzene includes an electrophilic substitution reaction that occurs between benzene and sulfuric acid. There are two …
Applications of Halogenation of Benzene
- Benzenesulfonic acid is used as an acid catalyst and to standardise dyes. A variety of pharmaceutical drugs are prepared as benzenesulfonate salts and are known as besilates or besylates. 4. Alkylation and Acylation of Benzene This reaction is popularly known as Friedel-Crafts reaction. The reactivity of haloalkanes gradually increases as you move up the periodic ta…
But This Alkylation Has A Couple of drawbacks. These Drawbacks Include
- There are chances of rearrangements
- The probability of multiple additions can’t also be ignored
- This is not applicable for benzenes with multiple electron-withdrawing groups.
Benzylic Position and Its Impact on Benzene Reactivity
- The benzene’s aromaticity is responsible for its resistance towards many of the reactions that alkenes typically can take part. However, chemists have found out ways to react benzene following various other methodologies. We begin our discussion of benzene reactions with processes that occur not on the ring directly, but at the carbon immediately bonded to the benze…