The rule for naming acids depends on whether the anion contains oxygen. If the anion does not contain oxygen, acid is named with prefix hydro- and suffix -ic to the root of element. If the anion does contain oxygen, the name is formed from root name of central element of anion or anion name, with a suffix of -ic or -ous.
What are the names of 10 different acids?
Acid Name Formula; Acetic Acid: CH3COOH: Acetylsalicylic Acid: HC9H7O4: Ascorbic Acid: HC6H7O6: Azelaic Acid: H2C9H14O4: Barbituric Acid: HC4H3N2O3: Benzilic Acid ...
What are the formulas of some common acids?
Acid Name Formula; Acetic Acid: CH3COOH: Acetylsalicylic Acid: HC9H7O4: Ascorbic Acid: HC6H7O6: Azelaic Acid: H2C9H14O4: Barbituric Acid: HC4H3N2O3: Benzilic Acid ...
What are the names of the strong binary acids?
pKaa quantitative measure of the strength of an acid in solution; a weak acid has a pKa value in the approximate range −2 to 12 in water, and a strong acid has a pKa value of less than about −2. Binary acids are certain molecular compounds in which hydrogen is combined with a second nonmetallic element; these acids include HF, HCl, HBr, and HI.
What are the rules for naming chemical compounds?
To name oxyacids:
- Omit “hydrogen”
- Start with the root name of the anion
- Replace – ate with – ic, or – ite with – ous
- Add “acid”
What are the 3 rules for naming an acid?
Rules for Naming the Acids To name an acid, we look at the -ide molecule in the covalent bond. We change the -ide ending to -ic. We add the prefix hydro to denote the hydrogen atom. We add the word acid at the end.
What are the rules for writing formulas for acids?
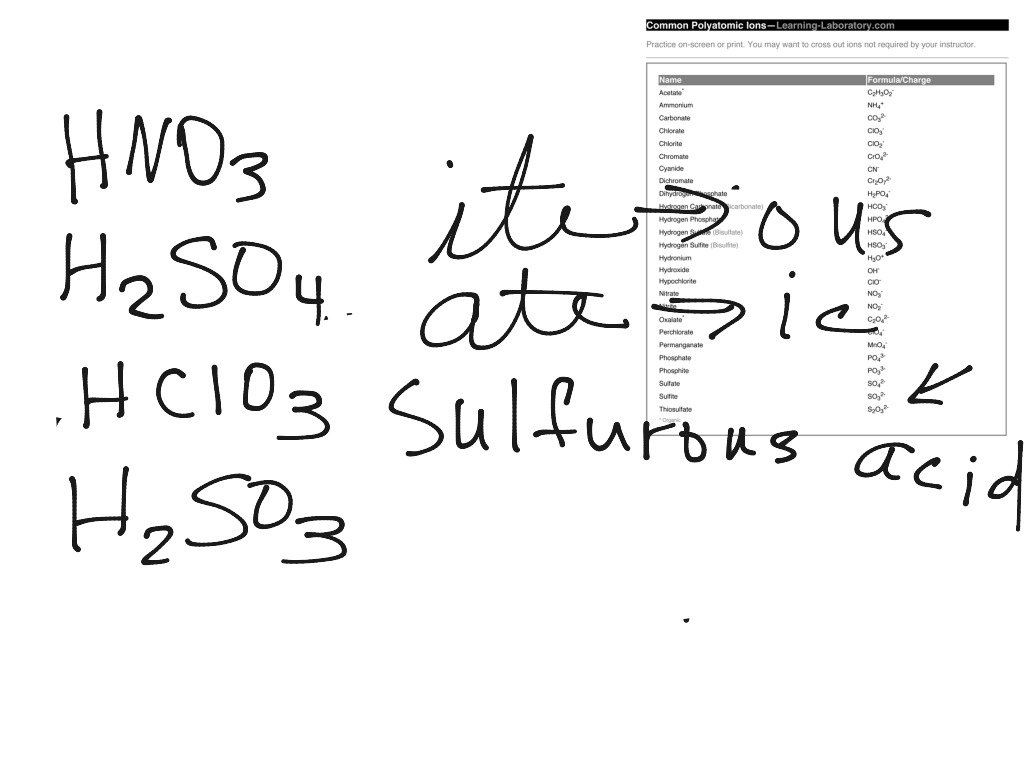
As a general rule the formula for an acid starts with hydrogen. If acid consists of just two elements, then it is named Hydro-_______-ic Acid. For example HCl is Hydrochloric Acid....You should memorize the following:H2SO4 = Sulfuric acid.HNO3 = Nitric Acid.HC2H3O2 = acetic acid.
What is a rule for naming binary acids?
All binary acids begin with the prefix hydro-, which precedes the name of any nonmetal other than hydrogen, in the compound. The name of this nonmetal is modified to end with the suffix -ic. Example: hydro. ic acid.
How do you name each acid?
Acids have their own nomenclature system. If an acid is composed of only hydrogen and one other element, the name is hydro- + the stem of the other element + -ic acid. For example, the compound HCl(aq) is hydrochloric acid, while H2S(aq) is hydrosulfuric acid.
What are the 3 rules for writing a chemical formula?
The rule for writing chemical formula is as follow: Firstly, write the symbols with positive charge valency first. Secondly, write the valency of each atom on the top of its symbol. Thirdly, divide the valency number by their highest common factor ignore the positive or negative radicle.
How do you name organic acids?
In general, carboxylic acids are named based on the number of carbons in the longest continuous chain, including the carboxyl group (-COOH). The suffix of this carbon chain is then replaced, as carboxylic acids always end in "-oic acid." An example is CH2O2, in which the longest continuous carbon chain is a methane.
What is Rule naming function?
In computer programming, a naming convention is a set of rules for choosing the character sequence to be used for identifiers which denote variables, types, functions, and other entities in source code and documentation.
Why are acids named so?
The word acid is derived from the Latin acidus/acēre, meaning 'sour'.
What are the rules for naming type 3 binary compounds?
Binary Covalent Compounds (Type III)The first element shown in the compound is named as the element (e.g., for CO2, first element is "carbon")The second element shown in the compound is named according to the anion name, ending in -ide (e.g., for CO2, the second element is named "oxide")More items...•
What is the easiest way to remember chemical names?
Here are some of the best (and worst) ways to memorize chemistry.Memorizing Chemistry Using Repetition.Memorizing Chemistry Using Mnemonic Devices.Using Memory Palaces To Memorize Chemistry.Using a Memory Palace To Memorize Numbers.
How can I memorize acids?
0:291:20MCAT Mnemonic: Strong Acids (Ep. 12) - YouTubeYouTubeStart of suggested clipEnd of suggested clipThese six strong acids. Is. So I brought no clean clothes you have so for sulfuric acid i4h iMoreThese six strong acids. Is. So I brought no clean clothes you have so for sulfuric acid i4h i brought for hbr no for nitric acid. Clean for HCl and close for perchloric acid.
How do you memorize acids?
0:264:29Memorize Strong Acids and Bases - YouTubeYouTubeStart of suggested clipEnd of suggested clipYou also need to recognize that they an ice 100% in water.MoreYou also need to recognize that they an ice 100% in water.
What are the rules for naming a binary covalent compound?
Naming Binary Covalent CompoundsName the non-metal furthest to the left on the periodic table by its elemental name.Name the other non-metal by its elemental name and an -ide ending.Use the prefixes mono-, di-, tri-.... to indicate the number of that element in the molecule.More items...
Which are rules used to name binary molecular compounds?
Naming binary molecular compounds is really quite easy. The first element is given its element name; the second is given its root (hydr, bor, carb, ox, fluor, etc.) followed by ide. For example, HCl is hydrogen chloride, and H2Se is hydrogen selenide.
How do you write formulas for binary acids?
3:139:47Binary Acids and Oxy-Acids: Naming and Formula Writing - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo to do this into a chemical formula. First thing you see you see hydro that means it's binary thatMoreSo to do this into a chemical formula. First thing you see you see hydro that means it's binary that means there's no oxygen. Now you see acid that also means just write an H automatically.
What do you put first when naming binary compounds?
Rule 1. The element with the lower group number is written first in the name; the element with the higher group number is written second in the name. Exception: when the compound contains oxygen and a halogen, the name of the halogen is the first word in the name.
What is the name of an acid?
Since all acids contain hydrogen, the name of an acid is based on the anion that goes with it. These anions can either be monatomic or polyatomic. The name of all monatomic ions ends in -ide. The majority of polyatomic ions end in either -ate or -ite, though there are a few exceptions such as the cyanide ion ( CN −). It is the suffix of the anion that determines how the acid is named, as displayed in the rules and table below.
What is the remainder of an acid?
The remainder of the acid (other than the H) is the anion after the acid dissolves. Organic acids are also an important class of compounds, but will not be discussed here. A binary acid is an acid that consists of hydrogen and one other element. The most common binary acids contain a halogen.
What is the name of the compound that releases hydrogen ions?
Acids are molecular compounds that release hydrogen ions. A binary acid consists of hydrogen and one other element. Oxoacids contain hydrogen, oxygen, and one other element. The name of the acid is based on the anion attached to the hydrogen.
What is the chemical name for a compound that contains one or more hydrogen atoms and produces hydrogen ions?
Acids. An acid can be defined in several ways. The most straightforward definition is that an acid is a molecular compound that contains one or more hydrogen atoms and produces hydrogen ions ( H +) when dissolved in water. Figure 2.12. 1: (A) Vinegar comes in a variety of types, but all contain acetic acid.
What is the root of sulfur?
Note how the root for a sulfur-containing oxoacid is sulfur- instead of just sulf-. The same is true for a phosphorus-containing oxoacid. The root is phosphor- instead of simply phosph-. Many foods and beverages contain citric acid.
How many suffixes are there for anions?
The three different suffixes that are possible for the anions lead to three rules:
Is an acid neutral or positive?
Like other compounds that we have studied, acids are electrically neutral. Therefore, the charge of the anion part of the formula must be exactly balanced out by the H + ions. Since H + ions carry a single positive charge, the number of H + ions in the formula is equal to the quantity of negative charge on the anion.
Naming Acids Definition
The naming of an acid can be done by following the IUPAC nomenclature. The naming of binary acids is based on the name of the anion with the prefix “hydro.”
Overview of Naming Acids
An acidic substance can produce H+ ions in its solution. Binary acids are formed by the combination of hydrogen with other elements. For example, HCl, HI, and HF are some examples of binary acids.
Common names of acids
Acids are one of the most common and widely known chemical compounds. The isolation of most simple carboxylic acids was from biological sources and the names of these acids were derived generally from the name of the source from which they are isolated. Some common names of acids are listed below:
Naming of binary acids
A binary acid is mainly composed of two parts: hydrogen and a non-metallic element. Here, hydrogen forms the positive part of the acid, e.g., H⁺ ion, whereas non-metals tend to accept electrons and form an anion to get an octet configuration. Like in HCl, H will be the positive part and Cl is the non-metallic element of this binary acid.
Naming of complex anion
The complex anion is usually a polyatomic anion that is composed of more than one atom and possesses one or more negative charge. Some common examples of polyatomic anions are listed below:
Rules for naming of complex acids
The complex acid is mainly composed of a hydrogen ion with a certain complex ion. According to IUPAC naming of complex acids, the name of a complex anion will determine the name of the acid.
Naming of organic acid
Organic acids are also known as carboxylic acids, as they have –COOH as the functional group. This functional group is bonded with the parent C chain of the organic compound. IUPAC suggested the suffix “-oic acid” for the naming of an organic compound. The name of organic acids is a combination of three parts: root word, prefix, and suffix.

CORE Concepts
Topics Covered in Other Articles
What Is An acid?
- An acid is a molecule or ion capable of donating a proton, known as a Brønsted–Lowry acid. An acid can also be defined as a molecule forming a covalent bond with an electron pair, known as a Lewis acid. Acids are always going to be ions or molecules. How is an acid a molecule? Well, all acids are made up ofnon-metals and a molecule is just another name for a covalent bond. Hydro…
How to Name Acids
- Naming Acids with Oxygen
Example number one nitric acid- 1. First – Identify the polyatomic anion HNO3→ NO3–realize that this is nitrate 2. Second – Write the name of the polyatomic ion NO3–= Nitrate 3. Third – Change the suffix -ate into -ic Nitrate → Nitric 4. Fourth – Add the word acid to the name (Nitric Acid) Ex… - Naming Acids without Oxygen
Example number one HCl – 1. First – Identify the monatomic anion HCl → Cl– 2. Second – Write the name of the monatomic anion Cl–= Chloride 3. Third – Replace the suffix -ide in the monatomic anion with -ic. Chloride → Chloric 4. Fourth – Add the prefix Hydro and the word Aci…