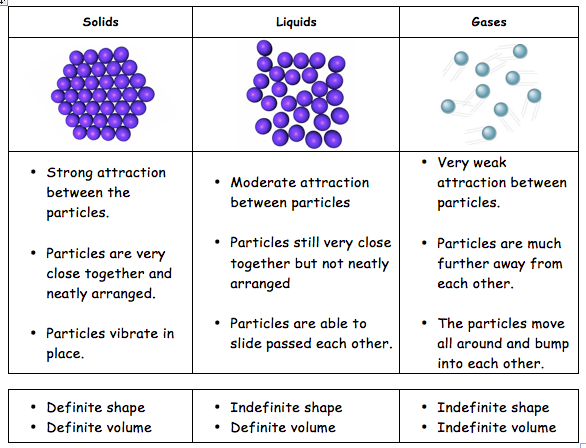
Different properties of solutions are as follows:
- It is a homogeneous mixture.
- Its particles are too tiny and have a diameter of less than 1 nm.
- The particles are not visible to naked eyes.
- Particles don’t scatter a beam of light passing through it and hence the path of the light is not visible.
- Solutes are inseparable from the mixture and do not sediment. A solution is...
- A solution is a homogeneous mixture.
- The constituent particles of a solution are smaller than 10-9 metres in diameter.
- Constituent particles of a solution cannot be seen by naked eyes.
- Solutions do not scatter a beam of light passing through it.
What are the three characteristics of a solution?
Characteristics. A solution is a homogeneous mixture of two or more substances. The particles of solute in a solution cannot be seen by the naked eye. A solution does not allow beams of light to scatter. A solution is stable. The solute from a solution cannot be separated by filtration (or mechanically).
What is one physical property of a solution?
Thus, solutions of solid solutes typically have a lower vapor pressure than the pure solvent. A related property of solutions is that their boiling points are higher than the boiling point of the pure solvent.
Which is a characteristic of a solution?
solution: Characteristics of Solutions Enter your search terms: The solute particles in a solution are generally of molecular size or smaller, much smaller than those in a colloid or a suspension.
What is characteristic of a solution?
Characteristics of a Solution A chemical solution exhibits several properties: A solution consists of a homogeneous mixture. A solution is composed of one phase (e.g., solid, liquid, gas).

What are the 3 types of solutions?
There are three types of solutions that can occur in your body based on solute concentration: isotonic, hypotonic, and hypertonic.
What are the types of properties of solution?
Colligative properties of a solution depend on only the total number of dissolved particles in solution, not on their chemical identity. Colligative properties include vapor pressure, boiling point, freezing point, and osmotic pressure.
What is a solution and its properties?
Answer: A solution is a homogeneous mixture of two or more substances. A solution has a solvent and a solute as its components. The component of the solution that dissolves the other component in it (usually the component present in larger amount) is called the solvent.
How many properties of solutions are there?
These properties are called colligative propertiesA characteristic of solutions that depends only on the number of dissolved particles.. Four important colligative properties that we will examine here are vapor pressure depression, boiling point elevation, freezing point depression, and osmotic pressure.
What are the properties of solutions answers?
Answers. Colligative properties are characteristics that a solution has that depend on the number, not the identity, of solute particles. In solutions, the vapor pressure is lower, the boiling point is higher, the freezing point is lower, and the osmotic pressure is higher.
What are the properties of solution Class 9th?
PROPERTIESA solution is a homogeneous mixture.The size of solute particle is very small ie less than 1 nm.The particle of a solution cannot be seen even with a microscope.The particle of a solution can pass through filter paper.A solution cannot be separated by filtration.More items...•
What are the properties of ideal solution?
Properties of an Ideal SolutionThe enthalpy of solution is zero. If the enthalpy of the solution gets closer to zero it is more likely to show an ideal behaviour. Δ m i x H = 0.The volume of mixing is also zero. Δ m i x V = 0.
What are the types of solutions?
13.1: Types of Solutions - Some TerminologySolutionSoluteExamplesgasgasair, natural gasliquidgasseltzer water (CO2 gas in water)liquidliquidalcoholic beverage (ethanol in water), gasolineliquidsolidtea, salt water2 more rows•Aug 25, 2020
What are some examples of solutions?
Sugar-water, salt solution, bras s, alloys, alcohol in water, aerosol, air, aerated drinks such as Coca-Cola etc. are the examples of solutions. When we work with chemistry, we generally prepare many types of solutions such as copper in water, iodine in alcohol etc.
What is concentration in chemistry?
Concentration of a solution refers to the amount of solute present in a given amount of solution. Amount of solute can be represented by its mass or volume. Concentration of a solution can be represented by various ways. Here we are providing you following three ways –
What are the types of solutions based on the presence or absence of water?
Types of solutions based on presence or absence of water: The solutions which have water as the solvent are called “aqueous” solutions. An example is a solution of salt and water. The substances that are dissolved in water are separated into individual components called ions.
What is a solution?
Solutions are homogeneous mixtures of two or more substances, containing very small sized solute particles. They do not scatter light; its particles cannot be seen by naked eyes. A solution is the basis for many products that are used in daily life like shampoos, glue, soda, and medicines. Molarity (number of moles per litre) ...
What is the maximum amount of solute that dissolves in a solvent at equilibrium?
In chemistry, the term “solubility” refers to the maximum amount of solute that dissolves in a solvent at equilibrium – this means that no more solute can be dissolved in the solvent in the set conditions (temperature, pressure). Such a solution is called a saturated solution.
What type of solution has the maximum amount of solute?
Types of solutions based on the amount of solute: • Unsaturated solution: The solution can take in more solute at a definite temperature. • Saturated solution: It is a solution that has the maximum amount of solute and cannot dissolve any more of it in the solvent at the present temperature and pressure.
What is a solute in chemistry?
Solute is that component (usually solid) that gets dissolved in a solvent to produce a homogeneous mixture. The amount of solute is usually less in quantity compared to the solvent. The concentration represents the amount of solute present in a chemical solution with respect to the amount of solvent. Some examples of solutes include sodium ...
How does temperature affect solubility?
Solubility is affected by temperature, pressure and molecular structure. An increase in temperature can increase the solubility of many compounds. • Supersaturated solution: In this type of solution, the amount of solute is in excess quantities than what the solvent can dissolve at that temperature and pressure.
What are some examples of solutes?
Some examples of solutes include sodium chloride, sugar (See figure 1), and carbon dioxide. Dissolution of a solute in a solvent to form a solution.
What are the different types of solutions?
Types of Solution. Liquid solutions, such as sugar in water, are the most common kind, but there are also solutions that are gases or solids. Any state of matter (solid, liquid, or gas) can act both as a solute or as a solvent during the formation of a solution.
What is the amount of solute in a solution?
The amount of solute in a given solution is called the concentration of a solution. The proportion of solute and solvent in solutions are not even. Depending upon the proportion of solute, a solution can be: 1 Diluted 2 Concentrated 3 Saturated
What is the concentration of a solution?
The Concentration of a Solution. The amount of solute in a given solution is called the concentration of a solution. The proportion of solute and solvent in solutions are not even. Depending upon the proportion of solute, a solution can be: Diluted.
What is the component that dissolves the other component called?
The component that dissolves the other component is called the solvent. 2. What is Solute? The component (s) that is/are dissolved in the solvent is/are called solute (s). Generally solvent is present in major proportion compared to the solute. The amount of solute is lesser than the solvent .
What are some examples of mixtures?
Mixtures are substances that consist of two or more types of matter. Air, soil, blood, etc. are different examples of mixtures. Based on the nature of the components and their distribution, mixtures are classified as homogeneous and heterogeneous mixtures.
Is sugar a gas or a solvent?
Here both the solvent and the solute are gases. Sugar syrup is a solution where sugar is dissolved in water using heat. Here, water is the solvent and sugar is the solute. Tincture of iodine, a mixture of iodine in alcohol.
Is air a solid or a gas?
Alloys and air are examples of solid and gaseous solutions, respectively. 3. Solution Examples. The following examples illustrate solvent and solute in some solutions. Air is a homogeneous mixture of gases. Here both the solvent and the solute are gases. Sugar syrup is a solution where sugar is dissolved in water using heat.
Components of Solution
Examples of Solutions
- Sugar-water, salt solution, brass, alloys, alcohol in water, aerosol, air, aerated drinks such as Coca-Cola etc. are examples of solutions. When we work with chemistry, we generally prepare many types of solutions such as copper in water, iodine in alcohol etc.
Types of Solutions
- Solutions can be divided into the following types of the basis of the quantity of solute in a solution – 1. Unsaturated Solution –The solution in which still we can add more solute at a given temperature is called an unsaturated solution. 2. Saturated Solution – The solution in which we cannot dissolve more solute in the solvent at a given temperature is called a saturated solution. …
Properties of A Solution
- A solution possesses the following properties – 1. A solution is a homogeneous mixture. 2. The constituent particles of a solution are smaller than 10-9metres in diameter. 3. Constituent particles of a solution cannot be seen by naked eyes. 4. Solutions do not scatter a beam of light passing through it. So, the path of the light beam is not visible...
Concentration of Solution
- The concentration of a solution refers to the amount of solute present in a given amount of solution. The amount of solute can be represented by its mass or volume. The concentration of a solution can be represented in various ways. The relative amount of solute and solvent present in a solution can be represented by different methods of expression. These ways are as follows. 1…
Conclusion
- We know that we use solutions in our daily life as they are an important part of our life. After going through the article we get all the necessary information related to the solution such as its properties, ways of expressing it and some common examples.