Facts about Solids, Liquids, and Gases
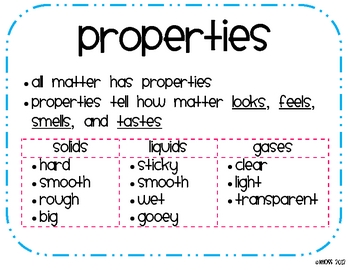
- Solids Solids have a fixed shape and volume. They are not compressible because their particles are tightly arranged together. ...
- Liquids Liquids assume the shape of the container, but not the volume. Although liquid particles can move around the container, they are still very close to one another, and there is not much space available. ...
- Gases Gases assume the shape and volume of the container. ...
- Solids – relatively rigid, definite volume and shape. In a solid, the atoms and molecules are attached to each other. ...
- Liquids – definite volume but able to change shape by flowing. In a liquid, the atoms and molecules are loosely bonded. ...
- Gases – no definite volume or shape.
What are the five essential properties of solids?
Some of the important characteristic properties of solids are:
- Solids have definite mass, shape and volume.
- Solids possess rigidity.
- Solids are almost incompressible.
- The density of solids is greater than that of liquids and gases.
- Most solids become liquids when heated, and this process is known as melting. ...
- Solids have short intermolecular distances and strong intermolecular forces.
What are some examples of properties of solids?
Properties of solids
- Electrical and thermal conductivity. As you read this lesson on your computer, you’re probably not thinking about the wires your computer uses to get the electrical power it needs to ...
- Malleability and ductility. ...
- Melting point. ...
- Solubility. ...
- Density. ...
- Summary. ...
- Key Concepts. ...
What are some properties of a solid liquid and gas?
What are some properties of solids liquids and gases? solid: Has a definite shape and volume. liquid: Has a definite volume, but take the shape of the container. gas: Has no definite shape or volume. change of state: When matter is converted from one of the three states (example: solid, liquid, or gas) to another state. What are properties of solids?
How are liquids different than solids or gases?
What are Liquids?
- Liquids have comparatively low inter-molecular force than solid.
- Liquids do not have fixed shape hence, takes the shape of the container in which it is kept.
- Liquids have fixed volume.
- Liquid as like solid cannot be compressed much.
- They have moderately high density. ...
- As the molecules are not tightly packed with each other they can flow.

What are the properties of solids liquids and gas?
A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. The change from solid to liquid usually does not significantly change the volume of a substance.
What are the 3 properties of a liquid?
Properties of LiquidsLiquids have fixed volume, but not fixed shape. ... Particles of Liquids are closely to each other (but not as close as solids)Liquid does not fill container completely like gases. ... Liquids are able to flow easily as particles are able to slide over each other.
What are the 3 properties of gas?
Gases have three characteristic properties: (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form. An internal combustion engine provides a good example of the ease with which gases can be compressed.
What are the properties of liquid and solid?
A solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape.
What are 3 properties of solids?
♣ Properties of Solids: Solid has a fixed shape and a fixed volume. Solid cannot be compressed. Solids have a high density. Force of attraction between the particles in a solid is very strong. The space between the particles of solids is negligible.
What are the 4 properties of gas?
Thus the gases are generally concerned with the relations among four properties, namely mass, pressure, volume, and temperature. The relationship between these physical properties of gases describes by Boyles, Charles, and Avogadro gas laws.
What are the 5 properties of liquid?
We hope that these cursory explanations of the nature of liquid characteristics provide a rudimentary understanding of and a curiosity about these five liquid properties: surface tension, consistency, viscosity, contact angle and density.
What are properties of solid?
Solid are characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does expands to fill the entire volume available to it like a gas .
What are two properties of solid?
The properties of the solid state are as follows:Solids have definite shape and volume.Solids possess rigidity.They cannot be compressed.The particles are closely packed.They cannot flow.
What are the 7 properties of gases?
Properties of GasesWhat are the Properties of Gases? Gasses do not possess any definite volume or shape. ... Compressibility. Particles of gas have huge intermolecular spaces in the midst of them. ... Expansibility. When pressure is exerted on gas, it contracts. ... Diffusibility. ... Low Density. ... Exertion of Pressure.
What are the 4 properties of solid?
1) A solid has a definite shape and volume. 2) Solids in general have higher density. 3) In solids, intermolecular forces are strong. 4) Diffusion of a solid into another solid is extremely slow.
What is liquid and its properties?
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, and plasma), and is the only state with a definite volume but no fixed shape.
What are 5 properties of liquid?
We hope that these cursory explanations of the nature of liquid characteristics provide a rudimentary understanding of and a curiosity about these five liquid properties: surface tension, consistency, viscosity, contact angle and density.
What is liquid and its properties?
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, and plasma), and is the only state with a definite volume but no fixed shape.
What are the 4 properties of fluids?
Temperature, density, pressure, and specific enthalpy are the thermodynamic properties of fluids.
What are 5 characteristics of a liquid?
liquid have a fixed volume but no fixed shape. liquids can be compressed. large pressure is required to compress them. liquids have lesser densities than solids. intermolecular forces of attraction is weaker than solids. they have considerable space between the particles.
What are the three states of matter?
The three states of matter are solid, liquid and gas . The particle model represents particles by small, solid spheres. It describes the arrangement, movement and energy of particles in a substance. The model can be used to explain the physical properties of solids, liquids and gases.
What are the particles in a diagram?
Particle arrangement and movement. The particles in the diagrams could be atoms, molecules or ions depending on the type of substance, eg ionic compounds, small molecules, giant molecules, and metals.
Does a particle have the properties of the material it is part of?
A single particle does not have the properties of the material it is part of. The properties of a substance are the properties of a huge number of particles together.
What are the physical properties of liquids?
What are physical properties of liquids? All liquids show the following characteristics: Liquids are almost incompressible. In liquids molecules are pretty close to each other. The molecules does not have lot of space between them. The molecules can not squeezed closer to one another.
Why is water an unusual compound?
Water is an unusual compound because it exists naturally on Earth in three states: solid, liquid, and gas. In which are the water molecules farthest apart?
Do liquids have a fixed volume?
Liquids have fixed volume but no fixed shape.They have fixed volume but they do not have fixed or definite shape. If you take 100 ml of water, pour water in a cup, it will take the shape of the cup. Now pour the liquid from cup to a bottle, the liquid has changed its shape and now it has taken the shape of bottle.

Properties of Solids
Properties of Liquids
- Atoms and molecules of the liquid are packed in a defined space in a semi-organized manner. They are able to move freely. They have weak forces of attraction as compared to solids and can easily change shape according to the shape of their container. Liquid molecules are simple and free to move. Agitation in the liquid result in the mixing of the m...
Properties of Gases
- Particles in gases are in random motion. The random motion of gases is explained through the kinetic molecular theory of gases. The collisions between gas molecules are perfectly elastic and that is the reason gases are mostly used in molecular studies. Gases do not have well-organized particles in a particular way. Molecules of ideal gases do not interact with each other. However, …
Concepts Berg
- What are the four main states of matter? There are four states of matter: 1. Solid 2. Liquid 3. Gases 4. Plasma What are examples of solid, liquid, and gas? When we take the ice and hold it in our hands, it is in the solid state. It then starts melting and converts into the liquid state. After that, it starts to vaporize which is a gas state. 1. Air, ethane, methane, and oxygen are examples of ga…