Types of homogeneous mixture?
- Solutions: A mixture formed when a substance (the solute) is dissolved in another substance (the solvent).
- Solute: the part of a solution that is being dissolved (usually the lesser part). Solvent: the part of a solution that dissolves the solute (usually the greater part). SOLUTION = SOLVENT + SOLUTE
What is the difference between heterogeneous and homogeneous grouping?
Therefore the key difference between homogeneous and heterogeneous is that homogeneous materials and mixtures have the same uniform composition and properties throughout whereas heterogeneous materials and mixtures do. Each of the layers is called a phase. Copyright Disclaimer Under Section 107 of the Copyright Act 1976 allowance is mad.
What are some examples of mixtures found in a kitchen?
- Pizza mass: this dough, which contains flour, yeast, water, salt, among other ingredients, is homogeneous as they are mixed uniformly.
- Bronze: this alloy is an example of homogeneous substances since it is composed of tin and copper.
- Milk : this mixture that we see in a uniform way is composed of substances such as water and fats.
What are some examples of mixtures and solutions?
Mixtures in two or more phases are heterogeneous mixtures. Examples include ice cubes in a drink, sand and water, and salt and oil. The liquid that is immiscible form heterogeneous mixtures. A good example is a mixture of oil and water. Chemical solutions are usually homogeneous mixtures.
What is an example of a heterogeneous solution?
What are 5 examples of heterogeneous mixtures?
- Concrete is a heterogeneous mixture of an aggregate: cement, and water.
- Sugar and sand form a heterogeneous mixture.
- Ice cubes in cola form a heterogeneous mixture.
- Salt and pepper form a heterogeneous mixture.
- Chocolate chip cookies are a heterogeneous mixture.

What are 2 homogeneous mixtures?
Examples of homogeneous mixtures include air, saline solution, most alloys, and bitumen. Examples of heterogeneous mixtures include sand, oil and water, and chicken noodle soup.
What are the 2 types of mixture?
There are two main categories of mixtures: homogeneous mixtures and heterogeneous mixtures.
What are the two types of heterogeneous mixtures?
There are two categories of heterogeneous mixtures: suspensions and colloids. Suspensions are mixtures that have larger particles that will separate into layers when left undisturbed. Colloids, by contrast, are substances that have particles mixed together that do not settle out but can be separated by chemical means.
What are the two types of homogeneous and heterogeneous?
There are two types of mixtures: heterogeneous and homogeneous. Heterogeneous mixtures have visually distinguishable components, while homogeneous mixtures appear uniform throughout. The most common type of homogenous mixture is a solution, which can be a solid, liquid, or gas.
What is another name for a homogeneous mixture?
a solutionAnother name for a homogeneous mixture is a solution. Solutions are made by dissolving a solute into a solvent. A solution of metals, for example, is called an alloy.
What are 2 types of solution?
Aqueous solution – When a solute is dissolved in water the solution is called an aqueous solution. Eg, salt in water, sugar in water and copper sulfate in water. Non-aqueous solution – When a solute is dissolved in a solvent other than water, it is called a non-aqueous solution.
What are types of homogeneous?
Homogeneous mixtures may be solids, liquids, or gases. Examples include steel, wine, and air. A homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. No matter where you sample the mixture, the amount and type of components is the same.
What are 3 heterogeneous mixtures?
Examples of Heterogeneous MixturesConcrete is a heterogeneous mixture of an aggregate: cement, and water.Sugar and sand form a heterogeneous mixture. ... Ice cubes in cola form a heterogeneous mixture. ... Salt and pepper form a heterogeneous mixture.Chocolate chip cookies are a heterogeneous mixture.More items...•
What are 2 heterogeneous mixtures in the kitchen?
A tossed salad is a heterogeneous mixture. A sandwich is a heterogeneous mixture. Ice cubes in a soda are a heterogeneous mixture. The ice and the soda are two distinct phases of matter (solid and liquid).
What is homogeneous mixture give an example?
For example, A mixture of salt and water, A mixture of water and sugar, These mixtures have the same composition of components throughout the mixture so both mixtures are examples of homogeneous mixtures.
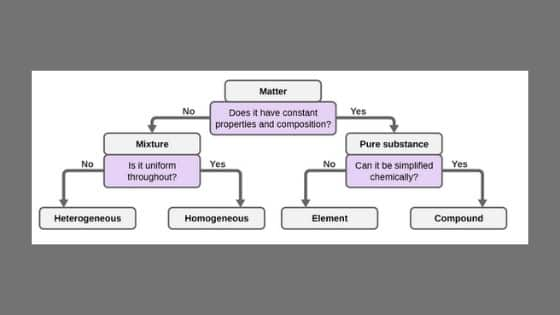
What are the two 2 classes of matter?
Matter can be broken down into two categories: pure substances and mixtures. Pure substances are further broken down into elements and compounds. Mixtures are physically combined structures that can be separated into their original components.
What are 5 homogeneous mixtures?
Examples of Homogeneous MixtureAir.Sugar water.Rainwater.Vinegar.Dishwashing detergent.Steel.Cup of Coffee.Mouthwash.More items...
What is a mixture give 2 examples?
Mixtures are substances that are made up of two or more different types of substances. Physical means can be used to separate them. A solution of salt and water, a combination of sugar and water, various gases, air, and so on are examples.
What are 2 mixtures we use everyday?
Some very common examples of heterogeneous mixtures found in daily life are a basket of vegetables, a box of toys, colored candies, salt and sugar, and salt and sand. Shampoo, vodka, vinegar, dishwashing liquid, and wine are homogeneous mixtures.
What are 2 types of pure substances?
Pure substances can be divided into two categories, elements and compounds. You already know that elements are pure substances that cannot be broken down into simpler substances. When elements combine, they form new substances called compounds.
What is a mixture of 2 liquids called?
When two liquids are able to get mixed to form a solution or are soluble in each other then they are called miscible.
What is a homogeneous mixture?
A homogeneous mixture is a type of mixture that has the same proportions of its components in a given amount of sample. Homogeneous mixtures can be solid, liquid, or gas. They have the same appearance and chemical composition throughout.
What are the two types of mixtures?
Mixtures can be broadly classified into two types: Homogeneous mixture and Heterogeneous mixture .
What is a colloidal solution?
Colloids: Colloidal solutions or Colloids are the mixtures in which microscopically dispersed insoluble particles of one substance are suspended in another substance, Not all the mixtures are colloids, The mixture where suspended particles don’t settle down at the bottom but get evenly dispersed into another substance . The size of the colloids ranges from 1nm to 1000 nm.
What are the different types of colloidal solutions?
Colloidal Solutions are divided into the following types: sol, emulsion, foam, aerosol, and gel.
What are the two major components of a solution?
Solutions have two major components, one is solute and the other one is solvent.
What is an alloy made of?
Alloys are formed when two or more metals are mixed together in some specific ratio. They usually are homogeneous mixtures. Example: Brass, bronze, steel, and sterling silver.
What is a mixture?
A mixture is defined as the combination of two or more substances that are combined physically. They can be separated by physical method. As there are no chemical changes involved, the components’ individual properties remain the same. In a mixture, every component has its own identity. During the formation of a mixture, there is no change in energy, and the Boiling point and the melting point of the mixture depend upon the characteristics of the constituents. Impure substances are also referred to as a mixture.
What are some examples of homogeneous mixtures?
Examples of Homogeneous Mixtures: Solid, Liquid and Gas. A chemical mixture combines two substances that maintain their own properties when combined. Heterogeneous mixtures are made up of a non-uniform composition, while homogeneous mixtures are made up of a uniform composition. For example, water and sand is a heterogeneous mixture — you can ...
Why is homogeneous mixture important?
Understanding homogeneous and heterogeneous mixtures is vital to building your knowledge of chemistry. Examples of homogeneous mixtures help reveal the remarkable scientific secrets that inform even the simplest parts of life. Next, take a look at some examples of physical properties of matter.
What is bronze made of?
Bronze - a mixture of copper and tin; bronze is a type of alloy, which is a metal created by two or more metallic elements
Is a solid homogeneous mixture a solution?
Solid Homogeneous Mixture Examples. Homogeneous mixtures are also known as solutions. When you think of a solution, you probably think of a liquid. However, many solids are also considered homogenous mixtures. There is a wide variety of solid homogeneous mixtures, from naturally occurring materials like stone to synthetic plastics.
Is milk homogeneous or heterogeneous?
Some people argue that homogenized milk — milk that has been treated by a machine to ensure that fat molecules are consistent throughout the liquid — is homogenous. While the substances (fat and water) will not separate in homogenized milk, it is technically a colloid. The fat is suspended rather than dissolved; therefore, milk is a heterogeneous liquid suspension of fats in water.
Is wine a mixture?
Wine - a homogeneous mixture (like all liquors); the science of making wine and liquor is based on employing ethanol and/or water as a solvent on various substances — charred oak for bourbon whiskey, for example, or juniper in gin — to create unique flavors.
Is a liquid a homogeneous mixture?
Many of the liquids you encounter every day are examples of homogeneous mixtures. These liquids include the beverages you drink, your bodily fluids and household cleaning materials.
What is homogeneous mixture?
A homogeneous mixture is a mixture throughout the solution in which the composition is uniform. The saltwater mentioned above is homogeneous due to the even distribution of the dissolved salt throughout the entire sample of saltwater.
What is a mixture?
What is Mixtures? Mixtures are formed when two or more substances (elements or compounds) mix together without participating in a chemical change.
Is water a gas or a mixture?
Water is a homogeneous mixture of nitrogen, oxygen and smaller amounts of other compounds in the gaseous materials. Stay tuned with BYJU’S to learn more interesting topics in Chemistry. Also, get various engaging and interactive video lessons to learn more effectively.
Is soft drink a homogeneous mixture?
In a homogeneous mixture, all the components are uniformly distributed and in the soft drink, we find components like sweetener, carbon dioxide and water forming a single phase. Therefore, a soft drink is a homogeneous mixture.
What is a liquid homogeneous mixture?
Liquid homogeneous mixture: a saline solution that is the mixture of water and salt.
What are some examples of mixtures?
For example; salt and water, where 2 different components are mixed to form a mixture.
What is an unsaturated solution?
An unsaturated solution is a solution in which a solvent can dissolve any more solute at a given temperature.
What is a non-aqueous solution?
Non-aqueous solution: When a solute is dissolved in a solvent other than water, then this type of solution is called a non-aqueous solution.
How many forms of solutions are there?
The solutions are of two forms, depending on the solvent if its water or not.
What are some examples of elements that do not mix?
For example; soil and water are 2 different components that do not mix and thus form a layer and can be seen through a naked eye.
Which type of mixture has only one phase?
The homogenous mixture/solution has only one phase that is solid, liquid and gas. Examples for the same are;
