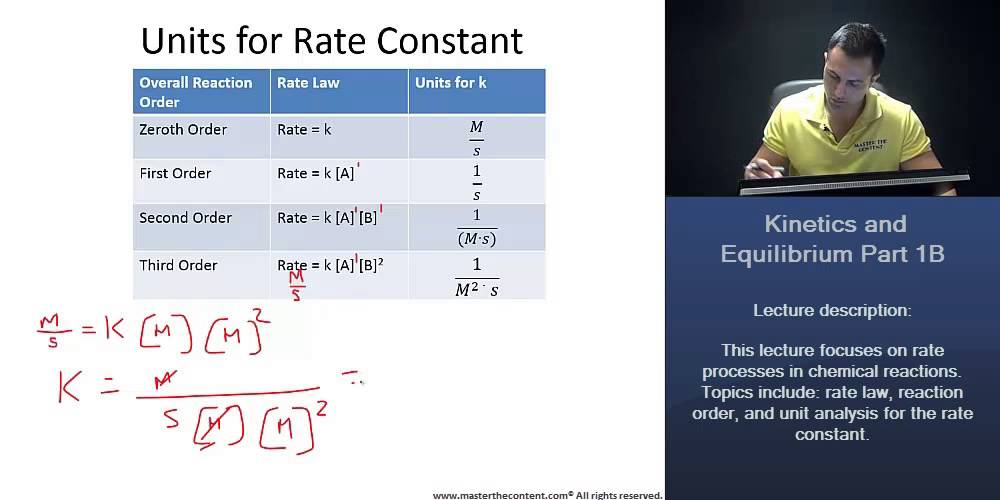
What are appropriate units for the rate constant k?
Rate Constant k has UNITS!
- Zero Order Reactions
- First Order Reactions
- Second Order Reactions
- Third Order Reactions
- n Order Reactions
What are the units of K in the following rate law?
What Are The Units Of K In The Following Rate Law? Rate = K [x]? For a given reaction with a rate=k [X]2 r a t e = k [ X ] 2, the rate of reaction is second order. Therefore, the units for k are 1M.
What are the units of the rate constant k for this reaction?
The units of the rate constant, k, depend on the overall reaction order. The units of k for a zero-order reaction are M/s, the units of k for a first-order reaction are 1/s, and the units of k for a second-order reaction are 1/ (M·s). See also what do leaves do for plants
Does a k constant have an unit?
k is a constant called the rate or spring constant (in SI units: N/m or kg/s2). When this holds, the behavior is said to be linear. What are the units of spring stiffness? Therefore, the spring constant k, and each element of the tensor κ, is measured in newtons per meter (N/m), or kilograms per second squared (kg/s2).
.PNG)
What are the units for the rate constant k for a second-order reaction quizlet?
A second-order rate constant is a proportionality constant for reactions having two reactants; it relates the rate of a reaction to the concentration of both reactants. Second-order rate constants have the unit M−1 s−1.
What is the rate constant of a second-order reaction?
Zero-Order ReactionsZero-OrderSecond-Orderrate lawrate = krate = k[A]2units of rate constantM s−1M−1 s−1integrated rate law[ A ] = − k t + [ A ] 0 [ A ] = − k t + [ A ] 01 [ A ] = k t + ( 1 [ A ] 0 ) 1 [ A ] = k t + ( 1 [ A ] 0 )plot needed for linear fit of rate data[A] vs. t1 [ A ] vs. t1 more row•Feb 14, 2019
What is the unit of second-order constant?
Solution : `"mol"^(-1)"L s"^(-1)` is the unit of rate constant for second order reaction.
How do you find k for a 2nd order reaction?
The order of the reaction is second, and the value of k is 0.0269 M-2s-1. Since the reaction order is second, the formula for t1/2 = k-1[A]o-1. This means that the half life of the reaction is 0.0259 seconds....1/Concentration(M-1)Time (s)3302 more rows•Mar 26, 2022
What is a 2nd order reaction?
Definition of second-order reaction : a chemical reaction in which the rate of reaction is proportional to the concentration of each of two reacting molecules — compare order of a reaction.
What are the units of a first-order rate constant?
Because the units of the reaction rate are always moles per liter per second, the units of a first-order rate constant are reciprocal seconds (s−1).
What are the units for the rate constant k?
Reaction Order and Rate Constant UnitsReaction OrderUnits of k(m+n)mol1−(m+n)L(m+n)−1s−1zeromol/L/sfirsts−1secondL/mol/s2 more rows
What is the unit of rate constant of 2nd order reaction if concentration in mole liter and time in second?
k=Lmol−1s−1.
How do you find the units of the rate constant k?
0:0812:14How To Determine The Units Of The Rate Constant K - Chemical KineticsYouTubeStart of suggested clipEnd of suggested clipSo rate is equal to k now there's a formula that can help you to find units of k. It's equal to mMoreSo rate is equal to k now there's a formula that can help you to find units of k. It's equal to m raised to the 1 minus n times t raised to the minus 1.
What is the unit of rate constant for third order reaction?
What is the unit of rate constant of the third order reaction? mol-2L2s-1 is the unit of third order reaction.
What is second-order reaction with example?
Reactions in which reactants are identical and form a product can also be second-order reactions. Many reactions such as decomposition of nitrogen dioxide, alkaline hydrolysis of ethyl acetate, decomposition of hydrogen iodide, formation of double-stranded DNA from two strands, etc.
What are the units of the rate constant?
The units of the rate constant, k, depend on the overall reaction order. The units of k for a zero-order reaction are M/s, the units of k for a first-order reaction are 1/s, and the units of k for a second-order reaction are 1/ (M·s). Created by Yuki Jung.
Why does order matter in rate law?
The order matters because the equations and graphs for the integrated rate law and half life are different depending on the overall order of the reaction. Hope that helps.
What is a negative reaction order?
Rate laws may exhibit fractional orders for some reactants, and negative reaction orders are sometimes observed when an increase in the concentration of one reactant causes a decrease in reaction rate.
Is the reaction order the same as the coefficient?
In some of our examples, the reaction orders in the rate law happen to be the same as the coefficients in the chemical equation for the reaction. This is merely a coincidence and very often not the case. Rate laws may exhibit fractional orders for some reactants, and negative reaction orders are sometimes observed when an increase in ...
What is a second order reaction?
A reaction is called a second order reaction when the overall order is two. Suppose if the reaction is as follows –. In these reactions rate is proportional to the square of the concentration of one reactant. The differential rate law for the above second order reaction can be written as follows –.
What is the sum of power of concentration of reactants in the rate law expression?
The sum of power of concentration of reactants in the rate law expression is called the order of that chemical reaction. Reactions can be first order reaction, second order reaction, pseudo first order reaction etc. depends on the concentration of the reactants. In this article we will discuss about second order reactions in detail.
What is the term for the half life of a reaction?
When chemical kinetics is used in pharma, it is called pharmacokinetics .
Why is half life important in pharmacokinetics?
Another vital application of half life in pharmacokinetics is that half – life for the drug reaction shows how tightly drugs bind to each ligand before it is undergoing decay. It is very important for drug design to know how tightly it binds with ligands. This was all about second order reactions.
