Type Ii Binary Ionic Compounds
- Type Ii Binary Ionic Compounds Definition These are the chemical compounds that are made up of one metal (cation) and one non-metal (anion), and the metal is a transition metal.
- Overview of Type Ii Binary Ionic Compounds ...
- Ionic Compounds: ...
- Nomenclature of Type II Binary Ionic Compounds: ...
- Examples of Type II Binary Ionic Compounds: ...
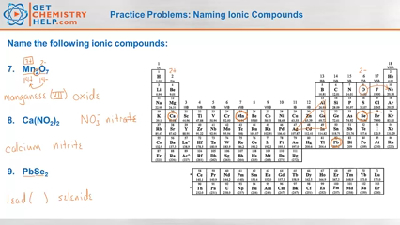
What is a type II binary ionic compound?
Type II: the metal can form two or more types of cations. Type II: they contain only non-metals. Type II binary ionic compounds have transition metals as cations. The following points must be kept in mind while naming these compounds:
What are the types of ionic compounds?
Generally, an ionic compound is made up of two elements – one metal and another non-metal. Such compounds can be further divided into three classes Type 1, Type2, and Type 3 binary compounds based on their composition. Type two ionic compounds are made up of transition metals as cations.
How to name ionic compounds using-IDE?
Naming Ionic Compounds Using -ide 1 H - Hydride 2 F - Fluoride 3 O 2- Oxide 4 S 2- Sulfide 5 N 3- Nitride 6 P 3- Phosphide
What is the difference between two-element ionic and polyatomic ionic compounds?
Two-element ionic compounds and polyatomic ionic compounds are equally common. Polyatomic ions are ions comprised of more than one atom. A good example is the ammonium ion made up of one nitrogen atom and four hydrogen atoms. Ions that are negatively charged are called anions, pronounced “an-ions.”
What is a Type II ionic compound?
0:1813:28Type II Binary Ionic Compounds - Naming and Writing FormulasYouTubeStart of suggested clipEnd of suggested clipWe have a metal that comes from the transition metals or the post transition metals except for aMoreWe have a metal that comes from the transition metals or the post transition metals except for a silver zinc or aluminum. And it's bonded to a nonmetal.
How Do You name Type 2 ionic compounds?
Simply put the name of the cation first and the name for the anion second. 2. Use Roman numerals after the name of the cation for Type II compounds (metals with more than one valence.
How do you tell if a compound is type 1 or 2?
0:5214:46Naming Type I & Type II Compounds - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo if you have a metal written first it's going to be a type 1 or type 2 compound it's going to beMoreSo if you have a metal written first it's going to be a type 1 or type 2 compound it's going to be named with a system or and talked about today.
What is a Type 3 ionic compound?
Compounds containing only non-metal elements are named using Type III binary compound rules. These compounds are always neutral (not ions which have charges), and consist of only two elements (see acid naming below for compounds containing only non-metal elements, but with more than two elements.
How do you name a type 1 ionic compound?
2:2810:44Type I Binary Ionic Compounds - Naming and Writing FormulasYouTubeStart of suggested clipEnd of suggested clipSimply name the metal followed by the nonmetal with an IDE ending. For example the metal here isMoreSimply name the metal followed by the nonmetal with an IDE ending. For example the metal here is called beryllium right it's beryllium.
How do you name a type 2 ionic compound zncl2?
The name of type 2 ionic compound ZnCl2 is Zinc (II) Chloride.
What are Type 1 and Type 2 compounds?
Type 1 binary ionic compounds are those in which the cation has only one form, or charge. Type 2 binary ionic compounds are those in which the cation can have multiple forms. Additionally, binary ionic compounds containing polyatomic ions have another distinct set of naming rules.
What is the difference between a Type I and Type II ionic compound How are they named differently?
There are two main types of ionic compound with different naming rules for each; Type I: compounds containing cations of main group elements and Type II: compounds containing cations of variable charge (generally transition metals). Below we will look at examples of each type to learn the rules for naming.
Is NaCl a Type 1 binary ionic compound?
Binary ionic compounds are compounds composed of monoatomic cations and monoatomic anions. For example, NaCl is a binary ionic compound composed of monoatomic cations Na+ and monoatomic anions Cl-.
What are the Type 1 metals?
The alkali metals are six chemical elements in Group 1, the leftmost column in the periodic table. They are lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
What are the 3 types of compounds?
Types of CompoundsMetal + Nonmetal —> ionic compound (usually)Metal + Polyatomic ion —> ionic compound (usually)Nonmetal + Nonmetal —> covalent compound (usually)Hydrogen + Nonmetal —> covalent compound (usually)
What are the 5 example of ionic compound?
Examples of ionic compounds in everyday life include table salt, baking soda, lye, Epsom salt, and bleach.
What is the difference between Type 1 and Type 2 ionic compounds?
Type 1 binary ionic compounds are those in which the cation has only one form, or charge. Type 2 binary ionic compounds are those in which the cation can have multiple forms. Additionally, binary ionic compounds containing polyatomic ions have another distinct set of naming rules.
How do you name a type 2 ionic compound AuCl3?
Gold trichloride | AuCl3 - PubChem.
How do you name a type 2 ionic compound FeCl3?
The chemical name of the compound FeCl3 is iron(III) chloride.
How can you tell if a compound is ionic 2?
0:158:15HOW TO NAME TYPE II IONIC COMPOUNDS - YouTubeYouTubeStart of suggested clipEnd of suggested clipRemember we said before an ionic compound has a metal and a nonmetal. But what we see now is type 2MoreRemember we said before an ionic compound has a metal and a nonmetal. But what we see now is type 2 compounds are made up of a metal. From groups 3 to 12 and 14. And they also consists of a nonmetal.
What is an ionic compound?
A compound where the cation and anion are held together by a force called ionic bond is known as an ionic compound. They are neutral compounds that are made up of charged species. Generally, an ionic compound is made up of two elements – one metal and another non-metal. Such compounds can be further divided into three classes Type 1, Type2, and Type 3 binary compounds based on their composition. Type two ionic compounds are made up of transition metals as cations. They have variable oxidation states and must be carefully named by taking their charge into consideration.
What is the bond between a positively charged ion and an ionic ion?
Chemical compounds whose ions are held together by electrostatic attractions (call ed ionic bond) between the positively and negatively charged ions are called ionic compounds. The electron released by an element (cation) is accepted by another element (anion) in order to attain octet configuration and hence stability. Although the ionic compound is composed of charged species, it is overall a neutral entity.
How to find the Roman numeral of a molecule?
By dividing the required total positive charge by the number of cations present in that molecule, the Roman numeral is obtained.
What is the root of the chemical symbol for chlorine?
Cl is the chemical symbol for chlorine. Its root is “chlor.” By adding the suffix “ide”, chloride is obtained.
Which type of metal can form only one type of cation?
Type I: the metal can form only one type of cation.
Is nickel oxide a neutral molecule?
Nickel (III) oxide is a neutral molecule, that is, the charges need to be balanced. This can be done by taking the least common multiple (LCM) of the two charges, that is, 3 and 2.
Is a cation an ion?
Since the cation is a transition metal ion, it can exhibit variable oxidation states. Care must be taken while determining the oxidation state of that element in that particular compound. A few examples are given.
What are the three types of ionic compounds?
They can be separated into three major classes. Type I : The metal can form only one type of cation. An example of type I binary ionic compound is calcium oxide (CaO). Type II : The metal can form two or more types of cations. An example of type III binary ionic compound is ferrous chloride (FeCl₂).
What are ionic compounds?
Compounds, where a cation and an anion are held together by ionic bonds , are called ionic compounds. They are neutral compounds that consist of charged species. Ionic compounds are made up of two elements – one metal and one non-metal. Such compounds can further be divided into three classes based on their composition as Type 1, Type2, and Type 3 binary compounds.
How to write a binary ionic compound?
To write the formula of Type 1 binary ionic compound, the following points must be considered: 1 If the cation belongs to Group 1, its charge is +1. 2 If the cation belongs to Group 2, its charge is +2. 3 In case the charge on the cation and that on the anion does not add up to zero, then the charge on the cation is written as a subscript of the anion and the charge on the anion is written as a subscript of the cation.
What is the bond between ions called?
Ionic compounds are chemical compounds where the ions are held together by electrostatic attractions (called an ionic bond) between the positively and negatively charged ions. Every element tends to attain a noble gas configuration. To do so, they either lose the electrons and become positively charged or they gain the electrons and become negatively charged. The positively charged particles are called cations. The negatively charged particles are called anions.
What is the charge of a cation in a group?
If the cation belongs to Group 1, its charge is +1.
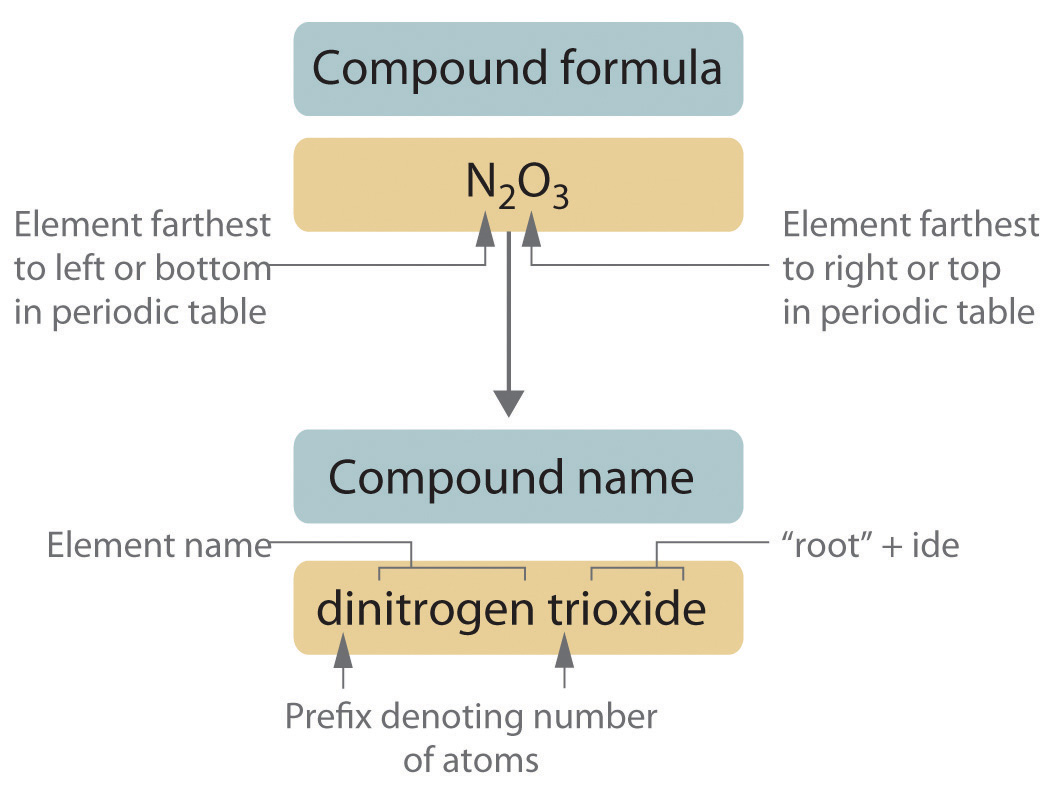
How to write compound names?
The name of the compound is written by combining the names of both the ions. The cation is named first and then the anion is named second.
What is the chemical formula for potassium sulfide?
Accordingly, 2 K atoms can give a total positive charge of +2 in order to balance the -2 charge on S. Therefore, the chemical formula of potassium sulfide is written as “K₂S”.
What is Ionic Compound?
The crystalline solids formed by neatly packed ions of opposite charge. Ionic compounds are usually formed when metals react with non-metals.
What is the structure of an ionic compound?
Ionic Compound Structure. The structure of an ionic compound depends on the relative sizes of the cations and anions. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Ionic solids are held together by the electrostatic attraction between the positive and negative ions.
What is the bond between metals and nonmetals?
The bond formed between them is known as the ionic bond. Due to the presence of oppositely charged ions, ionic compounds are held strongly by the electrostatic force of attraction.
How are ionic solids held together?
Ionic solids are held together by the electrostatic attraction between the positive and negative ions. For example, the sodium ions attract chloride ions and the chloride ion attracts sodium ions. The result is a three-dimensional structure of alternate Na + and Cl – ions. This is a crystal of sodium chloride.
How many electrons does magnesium need to complete its octet?
Therefore, it needs only one electron to complete its octet. It can gain this one electron from the electrons lost by magnesium atom to become magnesium ion. As two electrons are lost by magnesium atom while one chlorine atom can gain only one electron, two atoms of chlorine combine with one atom of magnesium to form magnesium chloride. ...
How many electrons does magnesium have?
For example reaction between magnesium and chlorine. The magnesium atom has two electrons in its outermost shell. By losing two electrons from its M shell its L shell becomes the outermost shell that has a stable octet. The nucleus of this magnesium atom still has twelve protons but the number of electrons has decreased to ten. So, a net positive charge is developed on this magnesium atom, giving a magnesium cation Mg 2+.
Why are ionic compounds hard to break?
Due to the presence of the strong force of attraction between the positive and negative ions, ionic compounds are solids and are hard to break. They generally break into pieces when pressure is applied, hence they are considered brittle.
