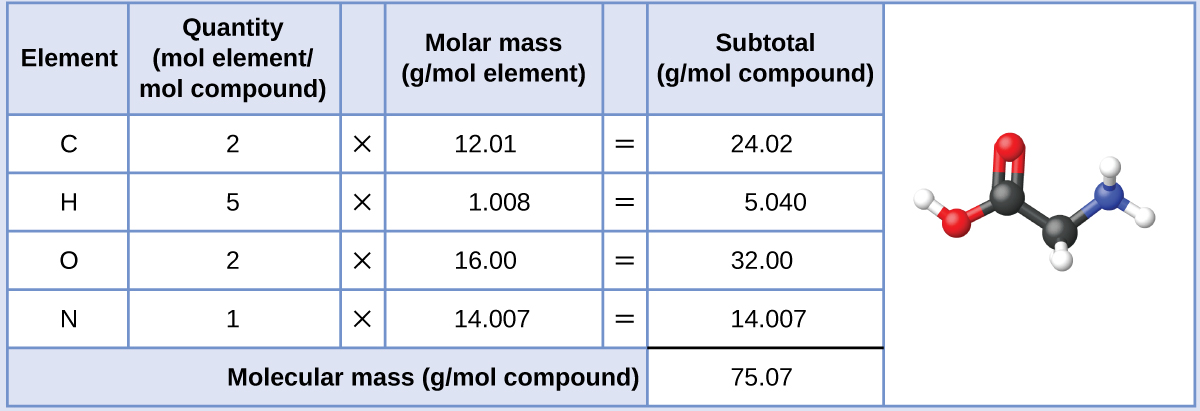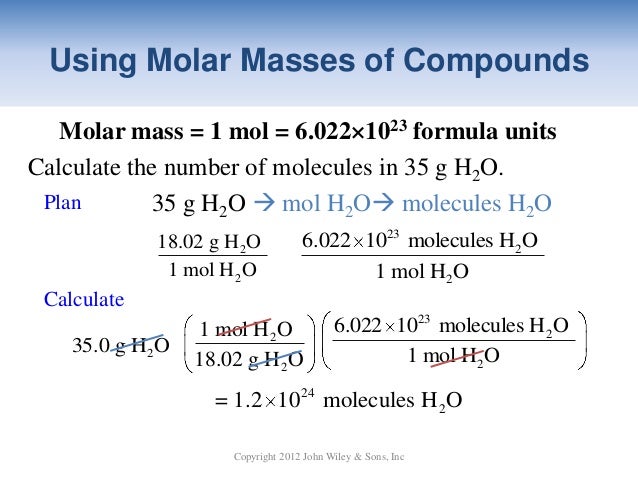
Understand molar mass. Molar mass is the mass (in grams) of one mole of a substance. Using the atomic
Atom
An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small; typical sizes are around 100 picometers (1×10⁻¹⁰ m, a ten-milliont…
| Known Information | Multiply By | Result |
|---|---|---|
| Mass of substance (g) | 1/ Molar mass (mol/g) | Moles of substance |
| Moles of substance (mol) | Avogadro's constant (atoms/mol) | Atoms (or molecules) |
| Mass of substance (g) | 1/Molar mass (mol/g) × Avogadro's constant (atoms/mol)) | Atoms (or molecules) |
How to calculate the moles of a substance?
In order to calculate the moles of a substance, you need to know the mass of the substance and its molar mass. Molar mass is the atomic weight in grams/mol. Example:
How do you find molar mass from atomic mass?
Understand molar mass. Molar mass is the mass (in grams) of one mole of a substance. Using the atomic mass of an element and multiplying it by the conversion factor grams per mole (g/mol), you can calculate the molar mass of that element.
What is the unit of measurement for molar mass?
The most commonly used unit for the molar mass of a substance is gmol -1. However, the SI unit for molar mass is kgmol -1 (or kg/mol). The molar mass can be calculated using the following equation. Mole or mol is the unit used to measure the amount of a substance.
Why is the molar mass important in chemistry?
Molar mass is used to determine the mass percentages of atoms present in a compound. Molar mass is very important in chemical reactions to find out the amounts of a certain reactant that has reacted or to find the amount of the product that can be obtained. Knowing the molar masses is very important before an experimental set up is designed.
How do you find the mass of one mole of a substance?
0:021:011.2.2 Calculate the mass of one mole of a species from its formula.YouTubeStart of suggested clipEnd of suggested clipSo molar mass is the mass of one mole of a substance in grams the unit for molar mass is grams perMoreSo molar mass is the mass of one mole of a substance in grams the unit for molar mass is grams per mole to the negative one. So let's try some examples the molar mass of NaCl sodium chloride.
What do you use to find the mass of a substance?
1 Answer. Multiply the number of moles of the substance by its molar mass in grams/mole (g/mol).
How do you find the mole of a substance?
0:145:541.2 Calculating amount of substance (in mol) - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe have amount of substance in moles is equal to the mass of the substance divided by its molar massMoreWe have amount of substance in moles is equal to the mass of the substance divided by its molar mass. The unit for mass is usually grams.
How do you find mass?
Mass is always constant for a body. One way to calculate mass: Mass = volume × density. Weight is the measure of the gravitational force acting on a mass.
How do you find the molar mass of a sample?
The molar mass is the mass of one mole of a sample. To find the molar mass, add the atomic masses (atomic weights) of all of the atoms in the molecule. Find the atomic mass for each element by using the mass given in the Periodic Table or table of atomic weights.
How do you find the mass of an unknown substance?
0:375:58Honors Chemistry Video 5.11 Molar Mass of an Unknown SubstanceYouTubeStart of suggested clipEnd of suggested clipSo all I have to do is I have to take this and divide by that number 273 grams divided by five pointMoreSo all I have to do is I have to take this and divide by that number 273 grams divided by five point two moles.
How do you find the mass of a substance in grams?
0:201:15How to Calculate Grams from Moles (Moles to Mass) - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe molar mass of oxygen is 32 grams per mole and we have ten point two moles of it. MultiplyingMoreThe molar mass of oxygen is 32 grams per mole and we have ten point two moles of it. Multiplying those two together we get three hundred twenty six point four grams.
Mole-Mass and Mass-Mass Calculations
From a given number of moles of a substance, calculate the mass of another substance involved using the balanced chemical equation.
Problem
How many moles of HCl will be produced when 249 g of AlCl 3 are reacted according to this chemical equation?
Test Yourself
How many moles of Al 2 O 3 will be produced when 23.9 g of H 2 O are reacted according to this chemical equation?
Problem
How many grams of NH 3 will be produced when 33.9 mol of H 2 are reacted according to this chemical equation?
Test Yourself
How many grams of N 2 are needed to produce 2.17 mol of NH 3 when reacted according to this chemical equation?
Answers
A calculation in which you start with a given number of moles of a substance and calculate the mass of another substance involved in the chemical equation, or vice versa.
How to find the molar mass of a chemical?
To find the molar mass, find the atomic mass of all the components of a chemical. You can either memorize it, or find all of the atomic masses located on the periodic table of elements. In this case, hydrogen has an atomic mass of 1, and oxygen has an atomic mass of 16. The equation is therefore: 1 (2) + 16 (1) = 18.
How to find molar mass?
Molar mass is the mass (in grams) of one mole of a substance. Using the atomic mass of an element and multiplying it by the conversion factor grams per mole (g/mol), you can calculate the molar mass of that element.
How many grams are in a mole of hydrogen?
This converts atomic units to grams per mole, making the molar mass of hydrogen 1.007 grams per mole, of carbon 12.0107 grams per mole, of oxygen 15.9994 grams per mole, and of chlorine 35.453 grams per mole. Some elements are only found in molecules of 2 atoms or more.
How to find the relative amount of each element in a compound?
Calculate the molar mass of each element in the compound. Multiply the element's atomic mass by the number of atoms of that element in the compound. This will give you the relative amount that each element contributes to the compound.
How many atoms are in a mole?
A mole is defined as the number of carbon atoms in 12 grams of the isotope carbon-12, which is roughly 6.022 x 10 23 atoms. This number is called Avogadro's number or Avogadro's constant.
What are the relative atomic masses of glucose?
The relative atomic masses of the elements in glucose are: carbon, 12.0107 g/mol; hydrogen, 1.007 g/mol; and oxygen, 15.9994 g/mol. Calculate the molar mass of each element in the compound. Multiply the element's atomic mass by the number of atoms of that element in the compound.
Why are atoms so small?
This article has been viewed 1,056,785 times. Atoms are too small to allow meaningful measurement of chemical substances. To work with meaningful amounts of substances, scientists group them into units called moles.
Why is it important to know the mole of a substance?
The mole is important because it allows chemists to work with the subatomic world with macro world units and amounts.
How many moles are in 20.0 grams of NaCl?
Since we know from the periodic table that the mass of one mole of sodium chloride is 58.44 grams, then; Therefore, the number of moles in 20.0 grams of sodium chloride is 0.34.
How to convert mass to mole
In chemistry, the molar mass of a chemical compound is defined as the mass of 1 mole (or 6.02214×10 23 particles) of the substance, expressed in grams.
Related calculators
Check out our other chemistry calculators such as Alcohol Dilution Calculator or Moles to Atoms Converter.
How to find moles in a solution?
How to Find Moles? How to find moles in the solution is to calculate how many molecules the solution contains. Also, to do this you need to know the volume of the solution and how many solutes has been dissolved in the solution. Furthermore, you also need to know the molar mass of the solute.
What is the molar mass of a molecule?
For example, the Chlorine (Cl) has a molar mass of 35.4530 g/mol in the same way Sodium (Na) has a molar mass of 22.9898 g/mol. Moreover, most of the molecules are made up of more than 1 element.
How many g/L is NaCl?
So, in this way the mass of one mole of NaCl is the mass of Na and mass of Cl: NaCl = Na + Cl. NaCl = 22.9898 g/L + 35.4530 g/L. Therefore, NaCl = 58.4538 g/L. Note: From compound to compound to a number of atoms in a molecule vary. For example, one molecule of H2O has two ...