
Isotopes are great resources for learning about the history of the earth. They can inform us about the climate and tell us how old a sample is. Isotope Formation Isotopes can either form naturally or through laboratory experiments. When isotopes form naturally it can occur through radioactive decay.
How do you identify isotopes?
How do you identify isotopes? Look up at the atom on the periodic table of elements and find out what its atomic mass is. Subtract the number of protons from the atomic mass. This is the number of neutrons that the regular version of the atom has. If the number of neutrons in the given atom is different, than it is an isotope.
What is the formula for isotopes?
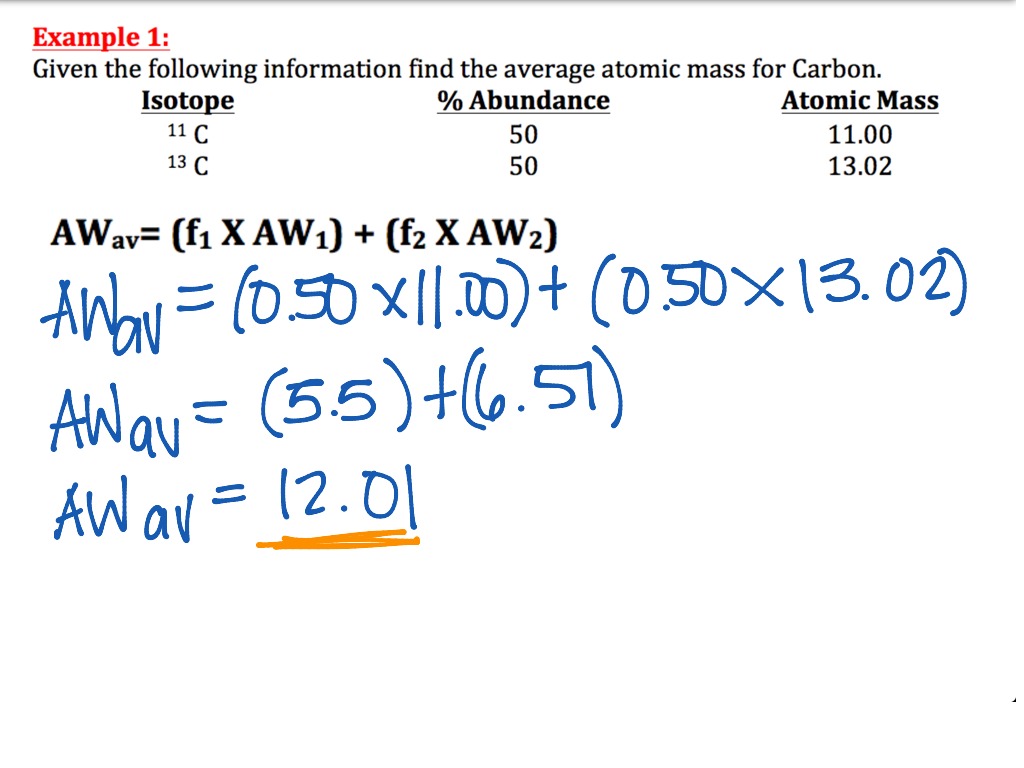
To calculate it, we have to take into account the abundance of each isotope, as shown in the formula below: Aᵣ = relative mass of isotope A × abundance of isotope A + relative mass of isotope B × abundance of isotope B. Let’s apply this formula on hydrogen.
What are the types of isotopes?
- Stable Isotopes
- Radioactive Hydrologic Applications Methods of Analysis:
- Decay Counting
- Mass Spectometry
- Gas Source
- Thermal Ionization (TIMS)
- Accelerator (AMS)
How to determine an isotope?
- The atomic number of an element is found above the elemental symbol within a box on the periodic table. ...
- The element with an atomic number of 74 is named tungsten.
- The number of protons present in an atom is defined by the element's atomic number. ...
What does an isotope tell you?
Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons. The number of protons in a nucleus determines the element's atomic number on the Periodic Table. For example, carbon has six protons and is atomic number 6.
What can isotopes be used for?
Stable isotopes can be used by measuring their amounts and proportions in samples, for example in water samples. Naturally-occurring stable isotopes of water and other substances are used to trace the origin, history, sources, sinks and interactions in water, carbon and nitrogen cycles.
What are 4 ways isotopes can be used in science?
Radioactive isotopes find uses in agriculture, food industry, pest control, archeology and medicine. Radiocarbon dating, which measures the age of carbon-bearing items, uses a radioactive isotope known as carbon-14. In medicine, gamma rays emitted by radioactive elements are used to detect tumors inside the human body.
Why are isotopes important in biology?
Isotopes are variations of chemical elements containing different numbers of neutrons. Because isotopes are recognizable, they provide an efficient way to track biological processes during experimentation. There are many potential uses for isotopes in experimentation, but several applications are more prevalent.
Which particles that make up an atom are involved in nuclear reactions quizlet?
A nuclear reaction involves the protons and neutrons, and chemical reactions involve the electrons.
Where are the electrons found in an atom?
Where Are Electrons? Unlike protons and neutrons, which are located inside the nucleus at the center of the atom, electrons are found outside the nucleus. Because opposite electric charges attract each other, negative electrons are attracted to the positive nucleus.
What is meant by a radioactive decay?
Radioactive decay is the emission of energy in the form of ionizing radiation. Ionizing radiation can affect the atoms in living things, so it poses a health risk by damaging tissue and DNA in genes.. The ionizing radiation that is emitted can include alpha particles.
What is a half-life in radioactive decay?
Half-life is the length of time it takes for half of the radioactive atoms of a specific radionuclide to decay. A good rule of thumb is that, after seven half-lives, you will have less than one percent of the original amount of radiation.
What is the purpose of 34 S?
While there is no enrichment of 34 S between trophic levels, the stable isotope can be useful in distinguishing benthic vs. pelagic producers and marsh vs. phytoplankton producers. Similar to 13 C, it can also help distinguish between different phytoplankton as the key primary producers in food webs. The differences between seawater sulfates and sulfides (c. 21‰ vs -10‰) aid scientists in the discriminations. Sulfur tends to be more plentiful in less aerobic areas, such as benthic systems and marsh plants, than the pelagic and more aerobic systems. Thus, in the benthic systems, there are smaller δ 34 S values.
What is isotope analysis?
Isotope analysis is the identification of isotopic signature, the abundance of certain stable isotopes and chemical elements within organic and inorganic compounds. Isotopic analysis can be used to understand the flow of energy through a food web, to reconstruct past environmental and climatic conditions, to investigate human ...
Why is it important to use stable isotope analysis?
The main advantage to using stable isotope analysis as opposed to stomach content observations is that no matter what the status is of the animal's stomach (empty or not), the isotope tracers in the tissues will give us an understanding of its trophic position and food source.
What are isotopes used for?
Archaeological materials, such as bone, organic residues, hair, or sea shells, can serve as substrates for isotopic analysis. Carbon, nitrogen and zinc isotope ratios are used to investigate the diets of past people; These isotopic systems can be used with others, such as strontium or oxygen, to answer questions about population movements and cultural interactions, such as trade.
What are the main effects of isotope hydrology?
The main effects that change the stable isotope composition of water are evaporation and condensation.
Why is isotope analysis important?
Isotope analysis has been particularly useful in archaeology as a means of characterization. Characterization of artifacts involves determining the isotopic composition of possible source materials such as metal ore bodies and comparing these data to the isotopic composition of analyzed artifacts.
What are the elements that are used in isotope ecology?
The main elements used in isotope ecology are carbon, nitrogen, oxygen, hydrogen and sulfur, but also include silicon, iron, and strontium.
Abstract
Carbon isotopes have been widely used as a tool to search for evidence of life in rocks from the early Earth. Many of the oldest rocks on Earth have been found to contain isotopically light reduced carbon, which many researchers have interpreted to have a biological origin.
Notes
This author’s research on this subject and preparation of this manuscript have been supported by the US National Science Foundation Earth Sciences Directorate through grants OCE-0550800 and EAR-0636056.
About this chapter
McCollom T.M. (2011) What Can Carbon Isotopes Tell Us About Sources of Reduced Carbon in Rocks from the Early Earth?. In: Golding S., Glikson M. (eds) Earliest Life on Earth: Habitats, Environments and Methods of Detection. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-8794-2_11
Abstract
In Europe, human-bear conflicts are an important topic for research and management. There are some scientific evidences that anthropogenic foods near settlements are promoting the development of conflict behavior by bears and are therefore causing human-bear conflicts.
References (0)
ResearchGate has not been able to resolve any citations for this publication.
How are oxygen isotopes different from other isotopes?
Oxygen isotopes are different atoms of oxygen, the difference being, as with any isotopes, that they have different number of neutrons in their nucleus, while the number of protons and electrons it the same for all isotopes.
How many neutrons are in an oxygen nucleus?
When there are fewer than eight neutrons or more than ten neutrons in an oxygen nucleus, that isotope is unstable and will decay radioactively. Fourteen radioisotopes of oxygen are known. O15 has the longest half life of 122 seconds and the rest all have short and very short half lives.
How many neutrons are in O16?
There are three stable isotopes of oxygen - O16 (with 8 neutrons), 017 (with nine neutrons and O18 (with ten neutrons. These occur naturally but O16 is by far the most common isotope (99.76%) with the balance being the other two isotopes.
How many protons does oxygen have?
Every oxygen atom, for instance can only contain eight protons, but it is possible for a number of neutrons to exist in the oxygen atom’s nucleus. Each different combination of neutrons with the eight protons forms an isotope of oxygen.
What do oxygen isotopes tell us?
What do oxygen isotopes tell us? They tell us that this super-important element to human beings exists primarily as a single isotope - oxygen 16 - and if it weren’t for that one isotope (of all the hundreds of other isotopes) you and I wouldn’t be here. We breathe it and our bodies are made of one of it’s most common compounds, water (H2O).
What is the dominant oxygen isotope?
The dominant oxygen is O-16 having 8-protons and 8-neutrons. But O-18 an isotope with 10-neutrons also exists. By discovering the ratio of O-16 to O-18 in a fossil scientists can obtain a reasonable estimate for the temperature at the time the organism existed. Scientists compare the ratio of isotopes in the fossil to the ratio in a standard to obtain a value called delta O-18. The equation to obtain the value is:
What is the shortest radioactive isotope?
Radioactive isotopes of oxygen with mass numbers from O-12 to O-24 have been characterized, all short-lived, with the longest-lived being O-15 with a half-life of 122.24 seconds, while the shortest-lived isotope is O-12 with a half-life of 580 (30) x 10^-24 seconds.

What Is An isotope?
Isotope Notation
- There are several ways scientists denote an isotope in writing. One way is to write the element name followed by the number of neutrons and protons added together. The number of protons plus neutrons also equals the mass number. For example, a carbon with 7 neutrons and 6 protons (instead of 6 neutrons and 6 protons) would be denoted as carbon-13. And a carbon with 8 neutr…
Common Isotopes and Their Uses
- All elementshave isotopes. However, some have more uses than others. Some of these isotopes are discussed below. Hydrogen Hydrogen has three main isotopes. These are protium, deuterium and tritium. Protium is 1H or hydrogen-1. Protium accounts for 99.98% of all hydrogen atoms. Deuterium (2H) and tritium (3H) comprise the last 0.02%. There are other isotopes, but these ar…
Fun Facts About Isotopes
- Every element has an isotope, they may just be very uncommon.
- Deuterium and tritium are the only isotopes with names. They refer to hydrogen with a single neutron and two neutrons respectively.
- The word isotope has Greek origin. ‘Iso’ means same and ‘topos’ refers to place.
- Isotopes are great resources for learning about the history of the earth. They can inform us a…
- Every element has an isotope, they may just be very uncommon.
- Deuterium and tritium are the only isotopes with names. They refer to hydrogen with a single neutron and two neutrons respectively.
- The word isotope has Greek origin. ‘Iso’ means same and ‘topos’ refers to place.
- Isotopes are great resources for learning about the history of the earth. They can inform us about the climate and tell us how old a sample is.
Isotope Formation
- Isotopes can either form naturally or through laboratory experiments. When isotopes form naturally it can occur through radioactive decay. That is an unstable isotope decays to form a new more stable isotope. Laboratories also make isotopes. Charged particles bombard an atom causing it to become an isotope. It may be an isotope of the same element or a different element.
List of Uses of Isotopes
- Here is a list of a few of the numerous uses for isotopes 1. Medical imaging 2. NMR Spectroscopy 3. Power source for space missions 4. Smoke detectors 5. Cancer therapy 6. Explosives detection 7. Nuclear reactors 8. Scientific research 9. Climate modeling 10. Radiocarbon dating 11. Sample dating
Radioactive Decay
- Radioactive is decay is the process by which an unstable isotope becomes a more stable isotope. A radioisotope refers to an unstable isotope. The new atom after decay may be the same element or different. During the process the atom losses energy to the environment in the form of ionizing radiation. Radiation types are alpha particles, gamma rays, and beta particles. An unstable isoto…