
Normal Soil Nitrate Levels
- Levels. The normal background level of nitrates in soil not fertilized or used for commercial crops ranges from 5 to 10 parts per 1 million (ppm).
- Soil Type. Nitrate levels are highest in soils that have finer textures, such as clay and silt, rather than those with rough textures, such as sand.
- Climate Conditions. Climate conditions can directly affect the amount of nitrate in soil. Excessive rainfall or standing water on soil can lead to leaching and denitrification, particularly in warm weather.
How are nitrates leached from the soil?
Nitrate-N can be leached from any soil if rainfall or irrigation moves water through the root zone. Denitrification Denitrification can be a major loss mechanism of NO 3--N when soils are saturated with water for two or three days. Nitrogen in the NH 4 +-N form isn’t subject to this loss. Management alternatives are available if ...
Is nitrate oxide the laughing gas?
Nitrous oxide is a safe and effective sedative agent that is mixed with oxygen and inhaled through a small mask that fits over your nose to help you relax. Nitrous oxide, sometimes called “laughing gas,” is one option your dentist may offer to help make you more comfortable during certain procedures. It is not intended to put you to sleep.
What is a high nitrate level?
What is considered high nitrate level? Although many aquarists run their tanks with extreme nitrate levels, the ideal is a maximum of 5 to 10 ppm. Levels of 20 to 50 ppm are too high. Freshwater tanks can be at the higher end, with marine fish-only setups at the lower end and reef tanks as near zero as possible.
Does lead nitrate an acid or a base?
The chemical compound lead(II) nitrate is the inorganic salt of nitric acid and lead. It is colourless crystal or white powder and a strong, stable oxidizer. Unlike most other lead(II) salts, it is soluble in water. Its main use from the Middle Ages under the name plumb dulcis, has been as raw material in the production of many pigments.
Why does nitrate leaching occur?
Nitrate is carried by soil water flow and can lead to leaching loss if there is enough movement of water out of the root zone. Nitrate leaching always occurs during the drainage season when precipitation and/or irrigation are higher than evaporation13,28.
How does nitrogen leaching happen?
Bacteria in the soil convert the ammonium nitrogen to nitrate- nitrogen which is highly soluble. So, the nitrate-nitrogen that is not captured by plant roots can be lost to the farming system. It is leached whenever the rain is such that water moves through the soil to groundwater.
How are nitrates leached?
The pathway for leached nitrate is down through the soil into groundwater, the groundwater eventually ends up in the river (receptor) and the river carries the nitrate to the estuary.
How do you stop nitrate leaching?
10 ways to reduce nitrogen leaching Use split application to adjust nitrogen availability to meet crop needs. Ensure balanced nutrition (P, K, S) to optimise nitrogen uptake. Develop a deep and extensive root system to capture available nutrients. Apply fertilisers and manure accurately across fields.
What are the causes of leaching?
Leaching happens when excess water, through rainfall or irrigation, takes water-soluble nutrients out of the soil. When water carries these nutrients away, they need to go somewhere.
How is nitrogen leached from soil?
Nitrogen can be lost from agricultural lands through soil erosion and runoff. Losses through these events normally don't account for a large portion of the soil N budget, but should be considered for surface water quality issues.
Are nitrates easily leached?
Nitrate is very mobile and easily leaches with water. Heavy rains can cause nitrates to leach downward in the soil below the root zone. Whether nitrates continue to leach downward, and into groundwater, depends on underlying soil and/or bedrock conditions, as well as depth to groundwater.
Why are nitrate susceptible to leaching in soils?
Among nutrient anions, nitrate is particularly easily leached because it shows negligible interaction with the negatively charged matrix of most topsoils and is, therefore, very mobile in the soil (see Section 5.2).
How can leaching be controlled?
Proper irrigation – giving your crop water when it needs it while not over-irrigating – is critical to preventing leaching.
How do you counteract nitrates in soil?
You can lay mulch over the soil with too much nitrogen to help draw out some of the excess nitrogen in the soil. In particular, cheap, dyed mulch works well for this. Cheap, dyed mulch is generally made from scrap soft woods and these will use higher amounts of nitrogen in the soil as they break down.
How do you reduce nitrogen loss in soil?
To reduce losses, use soil conservation best management practices (BMPs) including conservation tillage, no-till and cover crops; injecting or surface applying fertilizers in a band to facilitate passage through surface residue; and applying most nitrogen fertilizer as a mid-season sidedress when the established crop ...
What is leaching in the nitrogen cycle?
Leaching is the loss of nitrate N as water drains through the soil profile, moving out of the range of plant rooting systems.
Why is nitrogen leaching bad?
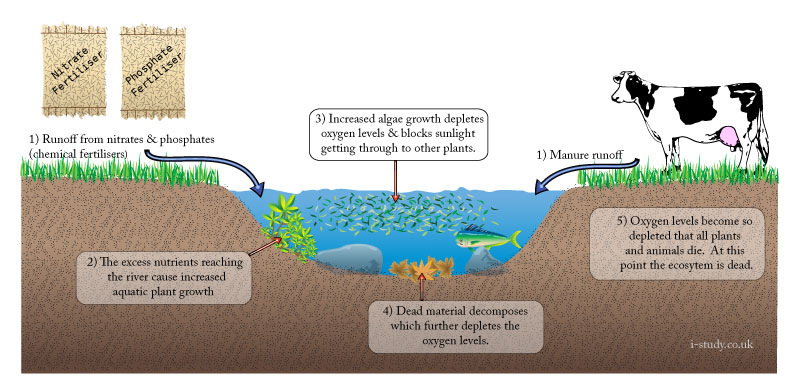
Nitrogen leaching losses into rivers and lakes can cause eutrophication resulting in excessive growth of aquatic weeds and algae, which can reduce fish populations and the recreational value of the water. Further, contamination of drinking water supplies by nitrate can cause health risks (Cameron et al. 2013).
How does nitrogen get into groundwater?
Nitrogen compounds in the fertilizer and organic matter are transported into the groundwater by percolating water from rainfall or from irrigation [3]. Hence, the nitrogen concentrations are typically high in agricultural areas [19]–[21].
Which form of nitrogen fertilizer has the greatest risk for leaching?
NitratesNitrate (NO3-) is primarily the form of nitrogen that is leached. Nitrates may originate from manures, the decay of plants and other organic materials or from fertilizers. Nitrate is very mobile and is easily moved by water.
How do nitrates leak into groundwater?
Under rainfall conditions, high levels of soluble nitrates (NO 3- –N) leak through soil and into groundwater with infiltration flow and then drain off with groundwater flow [ 35 ]. Our rainfall experiments also showed that only a small part of nitrates lost with surface water and high levels of soluble nitrates leak with infiltration flow. As shown in Fig 8, the average nitrate concentrations of groundwater increased continuously from 1st to the 8th experiments (0~300 hours), and then they began to decrease from the 9th experiment ( S3 Table ). None of the values during the 1st experiment exceeded the limit of nitrate concentration potability (10 mg/L) [ 8 – 10 ]. The nitrate concentrations increased as the experiments progressed. The mean values and most of the measured values remained below 10 mg/L prior to the 5th experiment. In contrast,as the experiments progressed, the mean concentrations were greater than 10 mg/L, and this value increased with time. Prior to the 7th experiment, all of the concentrations were greater than 10 mg/L. Next, experiments without fertilization were performed starting from the 8th experiment. However, the concentrations in the 8th experiment increased continually, and this experiment presented the maximum concentration of all of the experiments, with an average value of 14.75 mg/L. This result indicated that a reduction in fertilizer application would not lead to a rapid decrease of nitrate concentrations in groundwater. Because nitrate accumulation is the premise of leaching, a substantial amount of nitrate must have accumulated in the soil in the prior experiments. Then, the infiltration flow provides a carrier for the accumulated NO 3- –N in the soil profiles to move down, finally presenting the possibility of contaminating the groundwater [ 27, 61 ].
How does nitrate loss affect water?
A number of studies have shown that nitrate-nitrogen (NO 3- –N) loss through subsurface drainage is a major source of pollution for surface and groundwater bodies, thus threatening the water environment [ 28 – 31 ]. Nitrate is both soluble and mobile, it is prone to leaching through soil with infiltrating water, and it can persist in shallow groundwater for years [ 32 ]. Moreover, the hydrogeological settings, seasonal trends and anthropogenic activities are major factors that influence the mobility and accumulation of nitrates [ 33 ]. Under rainfall or irrigation conditions, high levels of soluble nitrates (NO 3- –N) leak through soil and into groundwater and then drain away with the groundwater flow. In the Weihe River Basin, groundwater is a streamflow recharge source in the upper reaches; in the middle reaches, one side of the river flow supplies the groundwater, and on the other side, the groundwater supplies the flow [ 34 ]. Therefore, nitrate leakage can cause nitrate pollution of groundwater; subsequently, the contaminated groundwater is likely to drain into rivers, resulting in further environmental damage to surface water [ 35 ].
What is non point source pollution?
Non-point source pollution caused by nitrogen from agro-ecosystems is a serious threat to water environments and has received increasing attention regionally and globally [ 12, 16 – 20 ]. Agricultural activities contributed to approximately 75% of non-point pollution, which accounted for approximately two-thirds of the total pollution, in the US [ 21 ]. Agriculture is a primary source of river and groundwater pollution in rural areas of the UK [ 22, 23 ]. The total nitrogen provided by agricultural non-point sources reached approximately 60% of the total water pollution in the Netherlands [ 24 ]. Approximately 94% of the nitrogen loading in 270 rivers was caused by non-point source pollution in Denmark [ 25 ]. Since the 1980s, nitrogen fertilizer consumption in China has substantially increased, and nitrate pollution of drinking water has become a serious problem [ 26 ]. Fan and Hao [ 27] summarized the primary factors for the accumulation and leaching of NO 3- –N in a soil profile and its potential contamination in surface and underground water in northern China.
Can nitrates cause pollution?
Therefore, nitrate leakage can cause nitrate pollution of groundwater; subsequently, the contaminated groundwater is likely to drain into rivers, resulting in further environmental damage to surface water [ 35 ].
Is nitrate a contaminant?
Nitrate is a common contaminant of surface water and groundwater and it can cause health problems in infants and animals as well as eutrophication of water bodies [ 1 – 7 ]. The World Health Organization and the U.S. Environmental Protection Agency have established a maximum contaminant level for nitrate of 10 mg/L as NO 3- –N in drinking water [ 8 – 10 ]. Many studies have shown that agricultural activities are a significant source of surface and ground water pollution due to long-term and excessive fertilizer use [ 7, 11 – 16 ].
Where does nitrate contamination occur?
Nitrate contamination occurs in surface water and groundwater, leaching into the soil and from there into the water supply from various sources. Irrigation water containing fertilizers is a common culprit as are septic systems, wastewater treatment plants, dairies and natural conditions.
How to manage nitrates in groundwater?
Meanwhile, there are also local efforts to handle nitrate. The Central Valley Water Board, for example, addresses nitrate in groundwater through four programs: 1 irrigated lands, minimizing discharge from irrigated agricultural lands 2 CV Salts, aiming to develop and implement a salinity and nitrate management program 3 groundwater quality protection strategy, providing a roadmap for future regulatory and control activities 4 dairy programs, focusing on control and abatement of nitrates to groundwater
Is it expensive to remove nitrates from water?
Tackling nitrate contamination directly is difficult and expensive. Nitrate is expensive to remove from drinking water supplies, especially in public and private systems that rely on untreated groundwater and do not have the necessary water treatment infrastructure.
Is nirtrogen fertilizer toxic?
It occurs naturally in soil and dissipates when the soil is extensively farmed. Thus, nirtrogen fertilizers are applied to replenish the so il. However, these nitrates can be toxic, especially when they enter the food chain via groundwater and surface water.
Is there a regulatory control on salinity?
There are some regulatory controls in place that address salinity, including nitrates, but it is generally agreed that a much more comprehensive approach is needed.
Is nitrate bad for plants?
Although a necessary nutrient for plants, high nitrate levels in people can harm the respiratory and reproductive system, ki dney, spleen and thyroid in children and adults. It is particularly harmful to infants. In California, nitrate is one of the most common groundwater contaminants.
What causes leaching?
Leaching is a natural process caused mainly by precipitation, acidification and nitrogen saturation. Human activity greatly influences some of these factors and the amount of leaching that occurs. Advertisement.
What are the factors that affect leaching?
Soil type affects leaching. Clay soils better retain nutrients than do sandy soils. If vegetation is present, more nutrients become tied up on site. Human activities including air pollution, some fertilization practices, harvesting of trees or other biomass, and mining all increase soil acidification.
What happens when nitrogen is saturated?
Nitrogen saturation increases acidification and the leaching of aluminum and nutrients. Positively charged ions of calcium, magnesium and potassium are drawn to the negatively charged nitrates as they leach out of the topsoil.
What causes soil to deplete nutrients?
Soil leaching leads to serious nutrient depletion. Soil leaching refers is the movement of nutrient elements from topsoil through the soil profile. Leaching causes significant nutrient losses, particularly in humid regions with high precipitation. Leaching is a natural process caused mainly by precipitation, acidification and nitrogen saturation.
What is the problem with soil leaching?
One serious soil-leaching problem relates to nitrogen, primarily in the form of nitrates. Nitrates naturally occur, but are often applied to croplands as fertilizer. Nitrates leach easily, depending on precipitation and soil type. Nitrogen saturation increases acidification and the leaching of aluminum and nutrients.
Why does soil leach?
Soil leaching occurs most directly because of precipitation. Whenever more water enters a system through precipitation than is lost through evaporation and transpiration, the excess water leaves the area by moving downward and collecting in groundwater or emerging in surface water.
Why is soil acidic?
Soils are naturally acidic or become increasingly acidic because of acid rain and air pollution, organic matter buildup or the presence of excess nitrogen. Acidic solutions dissolve compounds and displace nutrients.
Why is it difficult to remove nitrates from water?
Particularly, removal of nitrate from water is difficult task because it is chemically non-reactive in dilute aqueous solutions. Hence, the use of biological means for nitrate remediation offers a promising strategy to minimize the ill effects of nitrates and nitrites.
How does nitrogen affect agriculture?
Increased use of nitrogenous (N) fertilizers in agriculture has significantly altered the global N-cycle because they release nitrogenous gases of environmental concerns. The emission of nitrous oxide (N2O) contributes to the global greenhouse gas accumulation and the stratospheric ozone depletion. In addition, it causes nitrate leaching problem deteriorating ground water quality. The nitrate toxicity has been reported in a number of studies showing the health hazards like methemoglobinemia in infants and is a potent cause of cancer. Despite these evident negative environmental as well as health impacts, consumption of N fertilizer cannot be reduced in view of the food security for the teeming growing world population. Various agronomic and genetic modifications have been practiced to tackle this problem. Some agronomic techniques adopted include split application of N, use of slow-release fertilizers, nitrification inhibitors and encouraging the use of organic manure over chemical fertilizers. As a matter of fact, the use of chemical means to remediate nitrate from the environment is very difficult and costly. Particularly, removal of nitrate from water is difficult task because it is chemically non-reactive in dilute aqueous solutions. Hence, the use of biological means for nitrate remediation offers a promising strategy to minimize the ill effects of nitrates and nitrites. One of the important goals to reduce N-fertilizer application can be effectively achieved by choosing N-efficient genotypes. This will ensure the optimum uptake of applied N in a balanced manner and exploring the molecular mechanisms for their uptake as well as metabolism in assimilatory pathways. The objectives of this paper are to evaluate the interrelations which exist in the terrestrial ecosystems between the plant type and characteristics of nutrient uptake and analyze the global consumption and demand for fertilizer nitrogen in relation to cereal production, evaluate the various methods used to determine nitrogen use efficincy (NUE), determine NUE for the major cereals grown across large agroclimatic regions, determine the key factors that control NUE, and finally analyze various strategies available to improve the use efficiency of fertilizer nitrogen.
How does nitrogenous fertilizer affect the global N cycle?
Increased use of nitrogenous (N) fertilizers in agriculture has significantly altered the global N-cycle because they release nitrogenous gases of environmental concerns. The emission of nitrous oxide (N<sub>2</sub>O) contributes to the global greenhouse gas accumulation and the stratospheric ozone …. Nitrate and Nitrogen Oxides: Sources, Health ...
What are the factors that affect nitrogen?
What you should know 1 Numerous nitrogen (N) sources exist. Consider these when evaluating the N budget. 2 Soil type and climate greatly affect nitrogen loss from the soil system. 3 Because Minnesota has such diverse soils and climate, N cycle interpretations should be site-specific.
What is the main step in the production of commercial nitrogen fertilizers?
Commercial N fertilizers are also derived from the atmospheric N pool. The major step is to combine N2 with hydrogen (H2) to form ammonia (NH3). Anhydrous ammonia is then used as a starting point in the manufacture of other nitrogen fertilizers.
What is the nitrogen cycle?
Nitrogen cycle. Nitrogen exists in the soil system in many forms, and changes (transforms) very easily from one form to another. The route N follows in and out of the soil system is collectively called the nitrogen cycle (Figure 1). The nitrogen cycle is biologically influenced.
How is the nitrogen cycle influenced?
Biological processes, in turn, are influenced by prevailing climatic conditions along with a particular soil’s physical and chemical properties. Both climate and soils vary greatly across Minnesota and affect N transformations for the different areas.
How long does it take for nitrogen to decay?
Nitrogen exists in crop residues in complex organic forms and the residue must decay – a process that can take several years – before N becomes available for plant use. Soil organic matter. Soil organic matter is also a major source of N used by crops.
What can supply N to the soil system?
Any legume crop that’s left after harvest, including roots and nodules, can supply N to the soil system when the plant material is decomposed. Several nonsymbiotic organisms fix N, but N additions from these organisms are quite low (1 to 5 pounds per acre per year).
Why understand nitrogen?
Why understand N. Environmental and economic issues have increased the need to better understand the role and fate of nitrogen (N) in crop production systems . Nitrogen is the nutrient most often deficient for crop production in Minnesota, and its use can result in substantial economic return for farmers.
What causes nitrate leaching?
Large amounts of unused nitrogen in the soil, well-drained soil and heavy rainfall are the main causes of leaching. Reducing the amount of fertiliser alone won’t necessarily reduce residual nitrogen, but it could drastically diminish your yield. The only solution is to ensure your nitrogen application is tailored to the needs of your crop.
What causes leaching of nitrates?
Leaching occurs when mobile nitrate from the mineral nitrogen pool is washed out of the root zone by heavy rainfall. This is both inefficient and harmful to the environment. High concentrations of nitrates contribute to the eutrophication of watercourses, which can cause algae blooms to develop and deplete oxygen levels in the water.
Is nitrogen always lost?
But the nitrogen cycle is leaky by nature, and some nitrogen is always lost. Nitrate-based fertilisers and precision farming are your best weapons against nitrogen loss and lack of efficiency. TRY OUR QUIZ: Find out how you can improve your farm's nitrogen fertiliser efficiency.
