
To tell whether an unknown substance contains iron (II) nitrate or iron (III) nitrate, add a few drops of sodium hydroxide solution:
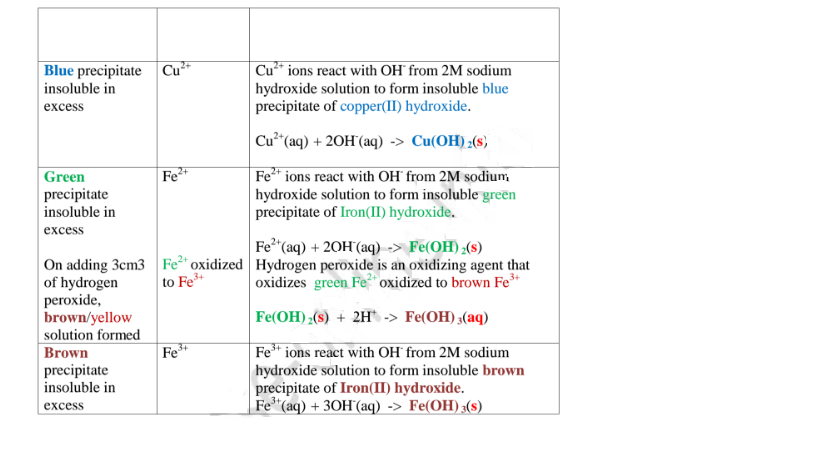
- if you get a green precipitate, the unknown substance is iron (II) nitrate
- if you get an orange-brown precipitate, the unknown substance is iron (III) nitrate
| Metal ion | Colour |
|---|---|
| Iron(II), Fe 2 + | Green - turns orange-brown when left standing |
| Iron(III), Fe 3 + | Orange-brown |
| Copper(II), Cu 2 + | Blue |
What is the color of iron II hydroxide?
The color of mercury (II) hydroxide is tan-brown. Does iron have any color? Pure iron is grey. Iron compounds have different colours, for example iron chloride is yellow, iron (II) hydroxide is green and iron (III) oxide is red-brown. What type of compound is iron II hydroxide? it is a compound!! What is the color of zinc II hydroxide?
Why does the green precipitate obtained from an iron (II) salt slowly turn brown?
Why does the green precipitate obtained from an iron (II) salt slowly turn brown when in contact with air? Due to oxidation. When iron (ll) is exposed to air, oxidation will occur and it will form iron (lll) salt which gives the brown colour.
What is the color of pure iron?
Pure iron is grey. Iron compounds have different colours, for example iron chloride is yellow, iron (II) hydroxide is green and iron (III) oxide is red-brown. What type of compound is iron II hydroxide?
What is the colour of precipitate with different ionic compounds?
Accoring to the anion or cation, colour can be vary. Chloride ion (anion) is common for both compounds. But their colours are different. However, ferrous chloride and ferric chloride are soluble in water. Lets consider lead +2 ion. PbI 2 is yellow colour precipitate and PbCl 2 is white precipitate. So when anion vary, color of preciptate also vary.
Which ions will give white precipitates with OH ions?
Which of these elements forms precipitates with metal ions?
What are the precipitates?
What are precipitates categorized as?
Why do carbonates have a variation in solubility?
How do colors help identify compounds?
What color are metal sulfides?
See 4 more
What is the colour of precipitate of iron?
The precipitated iron (II) hydroxide is dirty green in colour and is insoluble in excess of NaOH.
What will be the colour of precipitate formed by Fe2+ ion?
With Fe2+, a dark blue precipitate is formed.
What colour is iron II?
Iron(II) oxide is a black ionic solid that is also known as the mineral wustite, which is a black mineral. This mineral is found on Mars, but is not as common as the reddish compound iron(III) oxide, which is called hematite. Iron(II) oxide can be a greenish color if it has water molecules attached to it.
What precipitate does iron 3 form?
Caption. Iron (III) hydroxide precipitate formed by adding sodium hydroxide (NaOH) to a solution containing iron (III) ions. Iron (III) hydroxide (Fe(OH)3) is precipitated out of solution as a rust-brown gelatinous solid. Addition of sodium hydroxide is used as a test for positive metal ions in solution.
What is the colour of Fe2+ and Fe3+?
The difference between Fe2+ and Fe3+ is the Fe2+ has a pale green colour and turns violet when water is added to it. While Fe3+ forms blood-red when it reacts with thiocyanate ions.
How do you test for Fe2+ ions?
To tell whether an unknown substance contains iron(II) nitrate or iron(III) nitrate, add a few drops of sodium hydroxide solution: if you get a green precipitate, the unknown substance is iron(II) nitrate. if you get an orange-brown precipitate, the unknown substance is iron(III) nitrate.
What color is a iron 2 solution?
Iron(II) solutions contain the hexaaquoiron(II) ion [Fe(H2O)6]2+ which is a pale blue/green colour.
What is Fe2+ ion called?
Ferrous ion | Fe+2 - PubChem.
Why is Fe2+ green and Fe3+ yellow?
Iron usually exists in both Fe2+ as well as Fe3+ depending on the number of O2- vacancies and other point defects [2]. Fe3+ and Ni3+ in corundum yield yellow color whereas Fe2+ gives sapphire green color instead [3].
Why is iron +2 or +3?
Sometimes, iron will also lose one of the paired electrons from 3d orbital, leaving the entire 3d orbital filled with unpaired electrons (which provides a more stable configuration). In this case, its valency will be +3.
What is the difference between iron 2 and iron 3?
Summary – Iron II Chloride vs Iron III Chloride The key difference between iron II chloride and iron III chloride is that the Fe atom in iron(II) chloride chemical compound has +2 oxidation state whereas the Fe atom in iron(III) chloride compound has a +3 oxidation state.
Is Fe2+ soluble or insoluble?
Ferrous iron (Fe2+) is reasonably soluble at neutral pH in anoxic environments, but in the presence of oxygen aqueous Fe2+ is rapidly converted to the relatively insoluble ferric (Fe3+) oxide-hydroxide. Fe3+ is almost insoluble at neutral pH (10–18 M) but can be solubilized by acidification (10–6M at pH 3.0).
What is the colour of this precipitate?
Chemical testObservationTo solution W a small quantity of sodium hydroxide solution hydroxide solution is added and then in excess.A white precipitate is formed which remains insoluble.To a salt Z calcium hydroxide solution is added and then heated.A pungent smelling gas turning moist red litmus paper blue is obtained.2 more rows
What forms a yellow precipitate?
Yellow solid (precipitate) is produced when lead nitrate is added to the potassium iodide solution.
Which precipitate is white Coloured?
Therefore, barium carbonate, barium sulfate and barium sulfite are white precipitates.
What ion forms a white precipitate?
Precipitate testsMetal ionResult on adding NaOH or NH 3 solutionEffect of adding excess NaOH solutionaluminium, Al 3+white precipitatewhite precipitate dissolves and a colourless solution formszinc, Zn 2+white precipitatewhite precipitate dissolves and a colourless solution forms4 more rows
Qualitative Chemistry: Precipitation of Cations and Anions
6. The following precipitates might occur: Precipitate Color Precipitate Color PbCl 2 white Cu 3(PO 4) ⋅3H O bluish-white PbI 2 yellow Cu(OH) 2 blue Pb(OH) 2 white CuI brown PbSO 4 white Ag 2O brown, from Ag + and OH – Pb 3(PO 4)2 white AgCl white Ca(OH) 2 white Ag 3PO 4 yellow CaSO 4 white AgI yellow
Hydroxide precipitates - Testing for ions and gases - BBC Bitesize
Learn about the methods of testing for alkali metal ions and gases with BBC Bitesize GCSE Chemistry.
Solubility Rules and Identifying a Precipitate - Harper College
The Solubility Rules. 1. Alkali metal (Group IA) compounds are soluble. 2. Ammonium (NH 4 +) compounds are soluble. 3. Nitrates (NO 3-), chlorates (ClO 3-), and perchlorates (ClO 4-) are soluble. 4. Most hydroxides (OH-) are insoluble.. The exceptions are the alkali metal hydroxides and Ba(OH) 2. Ca(OH) 2 is slightly soluble. 5.
Precipitation Reaction - Examples & Definition - BYJUS
Precipitation Reaction is an chemical reaction occurring in aqueous solutions where two ionic bonds combine forming up insoluble salts. Learn more about the definition, examples & equations of precipitation reaction.
Which ions will give white precipitates with OH ions?
Magnesium, calcium, zinc, lead, aluminium ions will give white precipitates with OH - ions.
Which of these elements forms precipitates with metal ions?
Carbonate, sulfate, sulphite, phosphate, sulfide, chloride, bromide, iodide and more anions form precipitates with some metal ions. Now we consider about those precipitate of anions and those precipitates colours.
What are the precipitates?
Because precipitate is in the solid phase and deposited at bottom of the solution after kept it sometime to settle down.
What are precipitates categorized as?
Precipitates categorized as anions and cations : This section is bit different. Some anions form solutions with some cations. But with some cation, they form precipitates. As an example chloride ion can be given. Chloride ion with sodium ion form sodium chloride which is highly soluble in water. But, with lead +2 ion, it forms lead chloride (PbCl 2) white precipitate. In this section, we learn that type of variations too.
Why do carbonates have a variation in solubility?
Solubility of carbonates have a variation because there are soluble and insoluble carbonates. But when we study deeply about solubility of metal carbonates, most of the carbonates are insoluble in water.
How do colors help identify compounds?
Colours of precipitates help to identify compounds. We can decide which ions (cations or anions) are in the compound by comparing colours of different precipitates.
What color are metal sulfides?
Some metal sulfides are yellow colour. So adding some cations to aqueous sulfide ion solution, you can see yellow colour precipitates.
Which ion is present in the precipitation reaction?from bbc.co.uk
In the precipitation reaction which identifies the iron (III) ion as being present, the ionic equation is:
What is green precipitate?from bbc.co.uk
A green precipitate forms when dilute sodium hydroxide solution is added to a sample in solution. Identify the metal ion present in the original solution.
Why are my evergreens browning?from mrtreeservices.com
Besides disease, another major cause of evergreens browning is the weather conditions. The ground freezing in the wintertime can restrict the tree’s access to crucial water and nutrients that it normally draws from the soil.
What causes a tree to brown?from homeguides.sfgate.com
This condition is known as "winter burn," and it usually results from sharp drops in temperature and unusually cold temperatures.
Which group of metals are soluble hydroxides?from bbc.co.uk
Ions of group 1 metals (Li+, Na+ and K+) form soluble hydroxides. Therefore, they are identified using flame tests and not by adding sodium hydroxide solution.
Can sodium hydroxide be used to make magnesium ions?from bbc.co.uk
However, if excess sodium hydroxide solution is added: This means that using sodium hydroxide can give a positive result for aluminium ions, but it cannot distinguish between calcium and magnesium ions.
What is the test for anions and cations?
Precipitate tests. Many tests for anions and cations are precipitation reactions. When metal ions combine with the hydroxide ions (OH-) from either sodium hydroxide solution or ammonia solution, they form insoluble precipitates with characteristic colours. Method: dissolve a small quantity of the substance in water.
What is CCEA in chemical analysis?
Chemical analysis - (CCEA) Most elements are rarely found in their pure form. They are found chemically combined with other elements in compounds. Compounds are often found mixed with other compounds. Mixtures may be separated and analysed.
How to make a green precipitate?
add dilute sodium hydroxide solution... [1] ...and you would see a green precipitate [1]
What is silver nitrate used for?
Exam style question: Silver nitrate can be used to test for halide ions in aqueous solutions. Draw lines to match the ion to the correct colour of the precipitate formed with silver nitrate. [3 marks] answer choices. Chloride ------ White. Bromide ------ Cream.
What is a positive test for limewater?
Dilute acid added with pipette, connected with a tube to a test tube of limewater. Positive test if limewater goes cloudy.
Which ions will give white precipitates with OH ions?
Magnesium, calcium, zinc, lead, aluminium ions will give white precipitates with OH - ions.
Which of these elements forms precipitates with metal ions?
Carbonate, sulfate, sulphite, phosphate, sulfide, chloride, bromide, iodide and more anions form precipitates with some metal ions. Now we consider about those precipitate of anions and those precipitates colours.
What are the precipitates?
Because precipitate is in the solid phase and deposited at bottom of the solution after kept it sometime to settle down.
What are precipitates categorized as?
Precipitates categorized as anions and cations : This section is bit different. Some anions form solutions with some cations. But with some cation, they form precipitates. As an example chloride ion can be given. Chloride ion with sodium ion form sodium chloride which is highly soluble in water. But, with lead +2 ion, it forms lead chloride (PbCl 2) white precipitate. In this section, we learn that type of variations too.
Why do carbonates have a variation in solubility?
Solubility of carbonates have a variation because there are soluble and insoluble carbonates. But when we study deeply about solubility of metal carbonates, most of the carbonates are insoluble in water.
How do colors help identify compounds?
Colours of precipitates help to identify compounds. We can decide which ions (cations or anions) are in the compound by comparing colours of different precipitates.
What color are metal sulfides?
Some metal sulfides are yellow colour. So adding some cations to aqueous sulfide ion solution, you can see yellow colour precipitates.
