What determines the direction osmosis happens?
In osmosis, water moves from areas of low concentration of solute to areas of high concentration of solute.
What factors determine the rate and direction of osmosis?
The factors affecting the rate of osmosis include:Pressure.Temperature.Surface Area.Water Potential.Concentration gradient.
What determines the direction of water movement?
The net movement of water (osmosis) is in the direction of increased solute concentrations. An easy way to visualize this rule is simply that the net water movement is from an area of high water concentration (little dissolved solute) to an area of low water concentration (high levels of solute).
What is the direction of flow in osmosis?
The net flow of solvent through a semipermeable membrane is called osmosis (from the Greek osmós, meaning “push”). The direction of net solvent flow is always from the side with the lower concentration of solute to the side with the higher concentration.
What are the three types of solutions that determine the direction of osmosis?
Three terms—hypertonic, hypotonic, and isotonic—are used to describe whether a solution will cause water to move into or out of a cell: If a cell is placed in a hypertonic solution, there will be a net flow of water out of the cell, and the cell will lose volume.
What determines the direction of the diffusion?
What determines the direction of Diffusion? As Diffusion occurs from the area of high concentration to low concentration, we can say that the concentration gradient determines the diffusion direction.
What determines direction water will flow during osmosis across a semipermeable membrane?
The direction of passage of water through a semi-permeable membrane depends on the concentration of water molecules across the semi-permeable membrane.
Why does osmosis go from low to high?
Solvent moves from a region of higher concentration to a region of lower concentration. It can also be restated that osmosis involves movement of water across a semipermeable membrane separating two solutions of different concentrations from a region of lower solute concentration to higher solute concentration.
In what direction will osmosis water diffusion occur quizlet?
Diffusion is the movement of any substance going from high to low concentration. Osmosis is the movement of water, from high concentration of water to a low concentration of water through a selectively permeable membrane.
Does osmosis only occur in one direction?
It occurs in all directions. Osmosis is the movement of water or any other solvent from a region of low solute concentration to a region of high solute concentration.
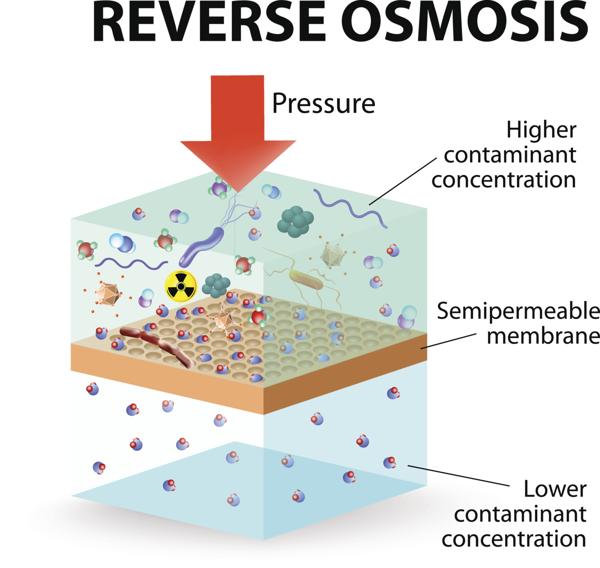
How can the direction of osmosis reverse?
In this process, greater pressure is applied, forcing the water to travel through the semipermeable membrane in opposite to natural osmosis. Reverse Osmosis works on the same principle as osmosis, but in the reverse direction. In this process direction of water flow is reversed by applying greater pressure.
Is osmosis and directional or directional?
Osmosis is Unidirectional in XYLEM transport and Osmosis is Bidirectional in PHLOEM transfer.. Mostly Osmosis is UNIDIRECTIONAL, because its the transport of water molecules from higher concentration to lower concentration through a semipermeable membrane.
What are 3 factors that affect the rate of osmosis?
Osmosis is the diffusion of water through a selectively permeable membrane from a higher concentration to a lower concentration. Several factors can affect the rate of osmosis. For example, temperature, particle size, and the size of the concentration gradient can all affect the rate of osmosis.
Which factors affect the rate of osmotic movement?
Which factors affect the rate of osmotic movement of water? The rate of osmosis varies with a number of factors, including temperature, pressure, and the difference in solute concentrations between two solutions separated by a selectively permeable membrane.
What factors affect the rate of diffusion and osmosis?
The greater the difference in concentration, the quicker the rate of diffusion. The higher the temperature, the more kinetic energy the particles will have, so they will move and mix more quickly. The greater the surface area, the faster the rate of diffusion.
What are the 4 factors that affect the rate of diffusion?
The rate of diffusion is affected by the concentration gradient, membrane permeability, temperature, and pressure. Diffusion takes place as long as there is a difference between the concentrations of a substance across a barrier.