Chemical Properties of Dilute Acids
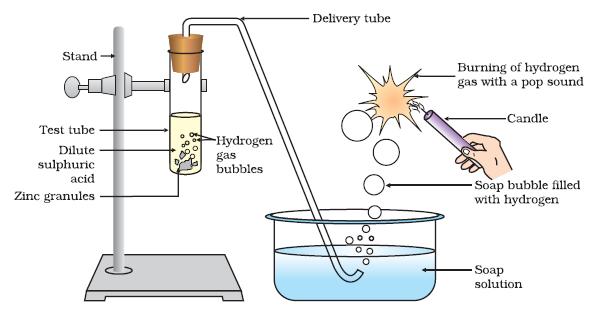
- Acids react with metals to release hydrogen gas Take some granules of zinc in one glass test tube and magnesium in another glass test tube and magnesium in another ...
- Acid reacts with a base (metallic oxide) to form salt and water 2HCl + ZnO → ZnCl2 + H2O Acid reacts with an alkali (hydroxide) to form salt and ...
- Acids reacting with Metals
What happens when dilute acids react with different metals?
The reactions of dilute acids with a variety of different metals can be investigated, small fresh samples of metals are placed in 2ml samples of dilute hydrochloric or sulfuric acid. The rate of reaction depends on the Concentration of the acid – if the acid is too dilute the reaction is very slow.
What are dilute acids?
What are Dilute Acids? Dilute acids contain a large amount of water. A concentrated acid can be diluted with the addition of water. All acids may be organic or inorganic, releasing hydrogen ions (H +) in water. Hence, acid is defined as “a substance which gives out hydrogen ions when dissolved in water”.
What happens when sodium reacts with dilute acid?
How does sodium react with dilute acid? Sodium reacts with dilute hydrochloric acid to form sodium chloride and hydrogen gas. Mixing with water produces sodium hydroxide.
How does potassium react with dilute acid?
Likewise, how does potassium react with dilute acid? Potassium reacts with dilute hydrochloric acid to give potassium chloride and hydrogen gas. Heating small pieces of Potassium in air results in the substance melting without any flame being seen and turning instantly into a mixture of potassium peroxide and potassium super oxide.
Examples of Acids
Properties of Dilute Acids
Chemical Properties of Dilute Acids
Does dilute acid react with sodium?
Reaction of Sodium with dilute Acid When sodium reacts with dilute hydrochloric acid, then sodium chloride is formed and hydrogen gas evolves.
What does dilute hydrochloric acid react with?
Dilute hydrochloric acid HCl reacts with metals to evolve gas along with the formation of corresponding metal salt. Dilute hydrochloric acid HCl reacts with metals to evolve gas along with the formation of corresponding metal salt.
What is the kind of reaction when acids are diluted?
Exothermic reaction occurs on dilution of acid or a base.
What does acids react with?
metalsIn general, acids react with metals to give salt and release hydrogen gas. In general, bases do not react with metals and release hydrogen gas.
Which metals react with dilute acids?
Dilute acids react with relatively reactive metals such as magnesium, aluminium, zinc and iron. The products of the reaction are a salt plus hydrogen gas. Here's a good way to remember it: MASH (M+A→S+H). In general, the more reactive the metal, the faster the reaction.
Which of the following most reactive in dilute acid?
Dimethyl amine, a secondary amine is the most reactive amine towards dilute hydrochloric acid.
How do metals react with dilute acid?
Metals react with dilute acids to form metallic salts and hydrogen gas. For example, Aluminium reacts with dilute hydrochloric acid to produce aluminum chloride and hydrogen gas.
How do acids react with water?
When you mix acid with water, it's extremely important to add the acid to the water rather than the other way around. This is because acid and water react in a vigorous exothermic reaction, releasing heat, sometimes boiling the liquid.
What happens when you add water to acid?
If you add water to acid, you form an extremely concentrated solution of acid initially and the solution may boil very violently, splashing concentrated acid. If you add acid to water, the solution that forms is very dilute and the small amount of heat released is not enough to vaporize and spatter it.
What acid reacts with salt and water?
Acids react with bases to form a salt and water.
Can acids react with acids?
1:232:26Reaction between 2 acids - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo this phosphoric acid is a stronger acid than nitrous acid so what that means is the phosphoricMoreSo this phosphoric acid is a stronger acid than nitrous acid so what that means is the phosphoric acid can donate a proton to the nitrous acid the nitrous acid has to act as a base and receive it.
Why do acids react with metals?
Atoms are always competing with each other for valence electrons. Hydrogen "wants" those electrons. So an active metal reacts with acids to produce hydrogen.
How do acids affect chemical reactions?
Acids are very useful in controlling and steering many chemical reactions . The products obtained from two very specific reagents can, in fact, change drastically by varying the acidity of the reaction environment.
Why are dilute acids labelled with a warning symbol?
Bottles of dilute acids are labelled with a warning symbol to indicate that they can irritate your skin or cause any other minor damage to health. It ensures that it will turn red or blistered if one of them makes contact with your skin.
What are some examples of acids?
Acids are very useful in controlling and steering many chemical reactions. Examples of acids are hydrochloric acid also known as muriatic acid and present in gastric juices. Sulfuric acid is also referred to as vitriol and several foodstuffs such as yogurt, vinegar and lemon.
Why are concentrated acids not stored in metallic containers?
Concentrated acids contain very little water. Acids are not stored in metallic containers because they react with metals and corrode them.
What is the pH of an acid?
Acid dissolve in water to form hydrogen ions (H + ). Acids are proton donors. Acids have a pH of less than 7.
How does water reduce the concentration of ions in a solution?
Water molecules reduces the concentration of ions in the solution by adding water to an acid or base. The concentration of H + ions decreases when an acidic solution is mixed with water, and the solution pH increases towards 7. The acid is less acidic.
Can a concentrated acid be diluted with water?
Dilute acids contain a large amount of water. A concentrated acid can be diluted with the addition of water.
What metals react with dilute acids?
Dilute acids react with relatively reactive metals such as magnesium, aluminium, zinc and iron. The products of the reaction are a salt plus hydrogen gas.
What is the name of the acid that dissolves lithium metals?
Lithium metal dissolves readily in dilute sulphuric acid to form solutions containing the aquated Li (I) ion together with hydrogen gas, H2. Lithium metals reacts slowly with water to form a colourless solution of basic lithium hydroxide (LiOH) and hydrogen gas (H2).

What Are Acids?
Understanding Dilute Acids
- Acids are corrosive in action. When we cook acidic food in brass or copper utensils they react with the metal and corrode them. Copper forms copper salt on combining with acid. Copper salts are poisonous and spoil the taste of the cooked food. This is why we do not store liquid food in metallic containers. Even tea should be served in copper cups. Metallic containers made from br…
Examples of Acids
- Acids are very useful in controlling and steering many chemical reactions. Examples of acids are hydrochloric acid also known as muriatic acid and present in gastric juices. Sulfuric acidis also referred to as vitriol and several foodstuffs such as yogurt, vinegar and lemon. Examples of some mineral acids and organic acids are shown in the table.
Properties of Dilute Acids
- Although there are many acids, there are several properties they have in common. Taste – Acids have a sour taste. Aqueous solution – Acids dissolve in water to form solutions which conduct electricity. Some acids completely ionize when they dissolve in water. These are called strong acids. The mineral acids are strong acids. Organic acids only ionize slightly when they dissolve i…
Chemical Properties of Dilute Acids
- 1. Acids react with metals to release hydrogen gas
Take some granules of zinc in one glass test tube and magnesium in another glass test tube and magnesium in another glass test tube. Pour dilute hydrochloric acid in one and sulphuric acid in the second. Reaction takes place and a gas is released in each. Test the gas for hydrogen by bri… - 2. Acid reacts with a base (metallic oxide) to form salt and water
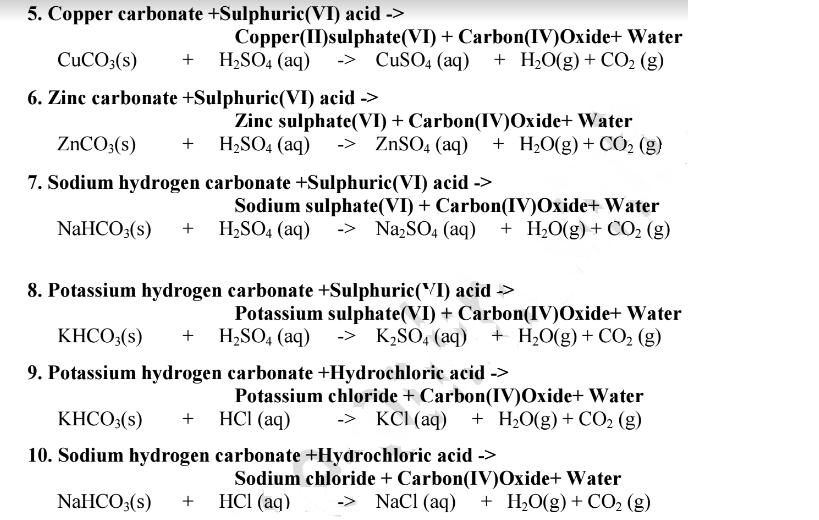
2HCl + ZnO → ZnCl2+ H2O Acid reacts with an alkali (hydroxide) to form salt and water. HCl + NaOH → NaCl + H2O In these Reactions acid neutralizes the base to form neutral substances like salt and water.